
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Fundamentals of Biophysics
About this book
Biophysics is a science that comprises theoretical plotting and models based on contemporary physicochemical conceptions. They mirror physical specificity of the molecular organization and elementary processes in living organisms, which in their turn form the molecular basis of biological phenomena. Presentation of a complete course in biophysics requires vast biological material as well as additional involvement of state-of-the-art concepts in physics, chemistry and mathematics. This is essential for the students to "perceive" the specific nature and peculiarity of molecular biological processes and see how this specificity is displayed in biological systems. This is the essence of the up-to-date biophysical approach to the analysis of biological processes.
Fundamentals of Biophysics offers a complete, thorough coverage of the material in a straightforward and no-nonsense format, offering a new and unique approach to the material that presents the appropriate topics without extraneous and unneeded filler material.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
Dynamic Properties of Biological Processes
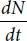
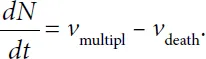
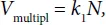
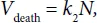
Table of contents
- Cover
- Half Title page
- Title page
- Copyright page
- Preface
- Chapter 1: Dynamic Properties of Biological Processes
- Chapter 2: Types of Dynamic Behavior of Biological Systems
- Chapter 3: Kinetics of Enzyme Processes
- Chapter 4: Distributed Biological Systems. Chaotic Processes
- Chapter 5: Mathematical Models in Ecology
- Chapter 6: Thermodynamics of Irreversible Processes in Biological Systems Near Equilibrium
- Chapter 7: Thermodynamics of Systems Far from Equilibrium
- Chapter 8: Physicochemical Principles of Biopolymer Structure
- Chapter 9: Intramolecular Dynamics of Proteins
- Chapter 10: Physical Models of Protein Dynamic Mobility
- Chapter 11: Energy Migration and Electron Transport in Biological Structures
- Chapter 12: Mechanisms of Enzyme Catalysis
- Chapter 13: Physicochemical Features of Biological Membranes. Ionic Equilibria
- Chapter 14: Passive Transport of Substances Across Membranes
- Chapter 15: Channels and Carriers. Active Transport
- Chapter 16: Transport of Ions in Excitable Membranes
- Chapter 17: Primary Processes of Energy Transformation in Photosynthesis
- Chapter 18: Energy Transformation in Biological Membranes
- Further Reading
- Index