
Physical Chemistry and Acid-Base Properties of Surfaces
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Physical Chemistry and Acid-Base Properties of Surfaces
About this book
The first part of this book looks at the consequence of chemical and topological defects existing on real surfaces, which explain the wettability of super hydrophilc and super hydrophobic surfaces. There follows an in-depth analysis of the acido-basicity of surfaces with, as an illustration, different wettability experiments on real materials. The next chapter deals with various techniques enabling the measurement of acido basicity of the surfaces including IR and XPS technics.
The last part of the book presents an electrochemical point of view which explains the surface charges of the oxide at contact with water or other electrolyte solutions in the frame of Bronsted acido-basicity concept. Various consequences are deduced from such analyses illustrated by original measurement of the point of zero charge or by understanding the basic principles of the electrowetting experiments.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
Wettability of an Ideal Surface: Overview
1.1. Wetting angle
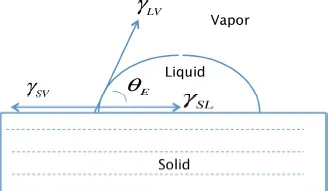
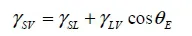
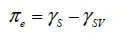
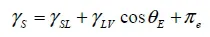
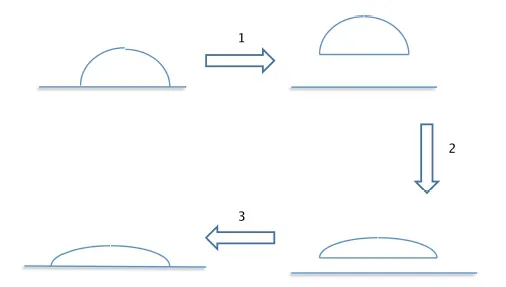
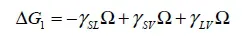
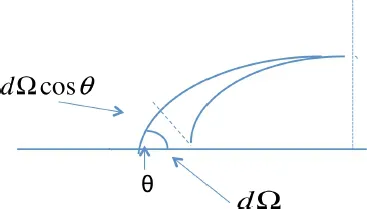
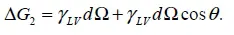
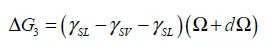
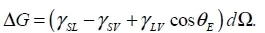
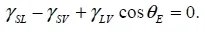
1.2. Adhesion effect
Table of contents
- Cover
- Table of Contents
- Title
- Copyright
- Introduction
- Chapter 1: Wettability of an Ideal Surface: Overview
- Chapter 2: Real Surfaces
- Chapter 3: Components of the Surface Energy
- Chapter 4: The Acid-Base Component in the Work of Adhesion
- Chapter 5: Experimental Determination through Wettability Measurements
- Chapter 6: Acid-Base Properties of Surfaces: Experimental Approaches
- Chapter 7: Oxide-Solution Interfaces: Surface Charges
- Chapter 8: Electrocapillarity Applications
- Conclusion
- Bibliography
- Index
- End User License Agreement