
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Physics of Magnetic Nanostructures
About this book
A comprehensive coverage of the physical properties and real-world applications of magnetic nanostructures
This book discusses how the important properties of materials such as the cohesive energy, and the electronic and vibrational structures are affected when materials have at least one length in the nanometer range. The author uses relatively simple models of the solid state to explain why these changes in the size and dimension in the nanometer regime occur. The text also reviews the physics of magnetism and experimental methods of measuring magnetic properties necessary to understanding how nanosizing affects magnetism. Various kinds of magnetic structures are presented by the author in order to explain how nanosizing influences their magnetic properties. The book also presents potential and actual applications of nanomaterials in the fields of medicine and computer data storage.
Physics of Magnetic Nanostructures:
- Covers the magnetism in carbon and born nitride nanostructures, bulk nanostructured magnetic materials, nanostructured magnetic semiconductors, and the fabrication of magnetic nanostructures
- Discusses emerging applications of nanomaterials such as targeted delivery of drugs, enhancement of images in MRI, ferrofluids, and magnetic computer data storage
- Includes end-of-chapter exercises and five appendices
Physics of Magnetic Nanostructures is written for senior undergraduate and graduate students in physics and nanotechnology, material scientists, chemists, and physicists.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
PROPERTIES OF NANOSTRUCTURES
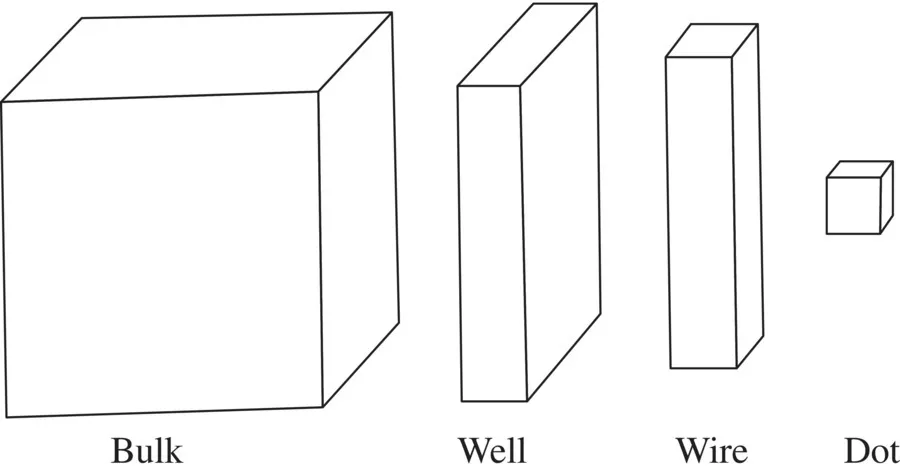
1.1 COHESIVE ENERGY
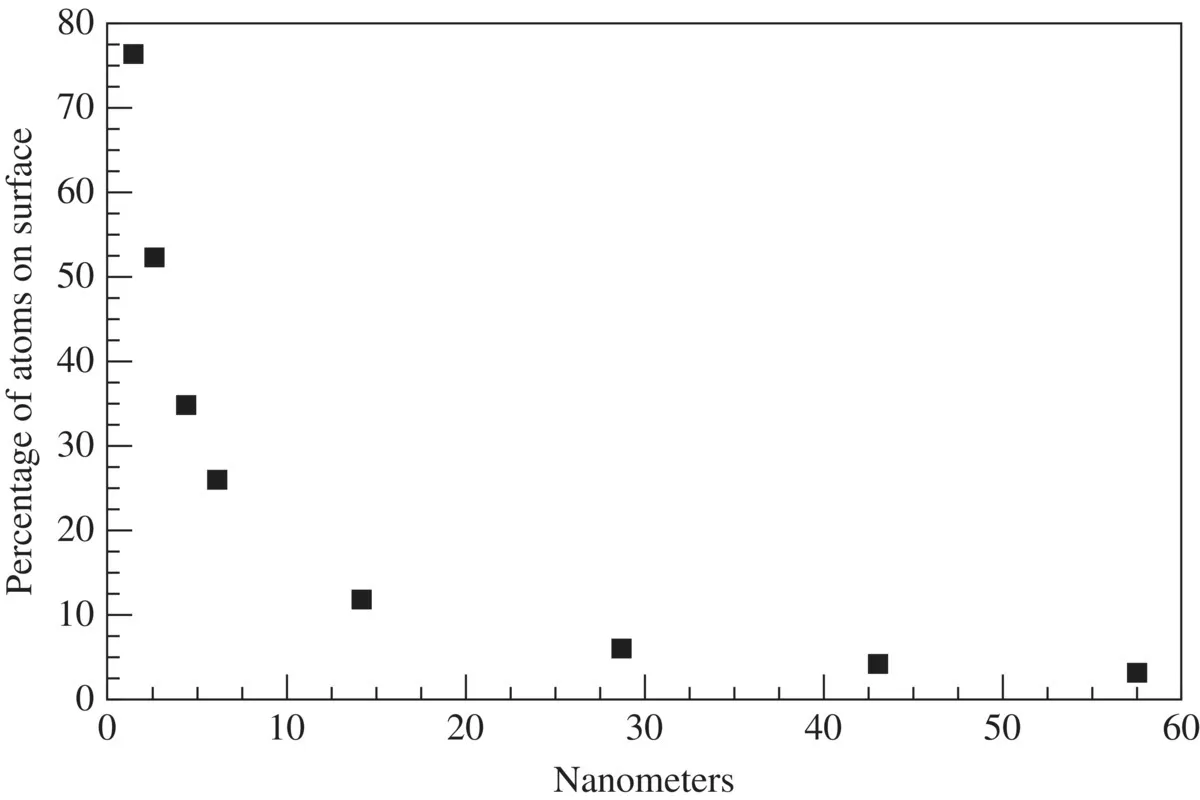
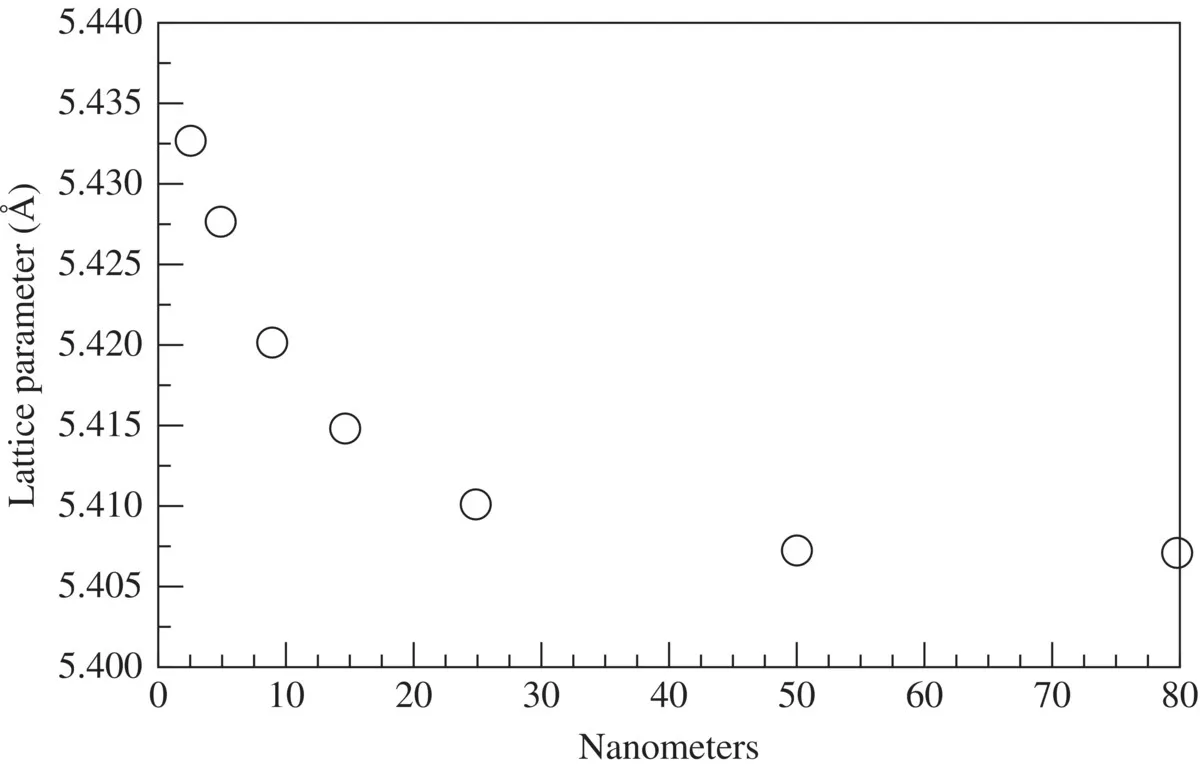
Table of contents
- COVER
- TITLE PAGE
- TABLE OF CONTENTS
- PREFACE
- ACKNOWLEDGMENT
- 1 PROPERTIES OF NANOSTRUCTURES
- 2 THE PHYSICS OF MAGNETISM
- 3 PROPERTIES OF MAGNETIC NANOPARTICLES
- 4 BULK NANOSTRUCTURED MAGNETIC MATERIALS
- 5 MAGNETISM IN CARBON AND BORON NITRIDE NANOSTRUCTURES
- 6 NANOSTRUCTURED MAGNETIC SEMICONDUCTORS
- 7 APPLICATIONS OF MAGNETIC NANOSTRUCTURES
- 8 MEDICAL APPLICATIONS OF MAGNETIC NANOSTRUCTURES
- 9 FABRICATION OF MAGNETIC NANOSTRUCTURES
- APPENDIX A A TABLE OF NUMBER OF ATOMS VERSUS SIZE IN FACE CENTERED CUBIC NANOPARTICLES
- APPENDIX B DEFINITION OF A MAGNETIC FIELD
- APPENDIX C DENSITY FUNCTIONAL THEORY
- APPENDIX D TIGHT BINDING MODEL OF ELECTRONIC STRUCTURE OF METALS
- APPENDIX E PERIODIC BOUNDARY CONDITIONS
- INDEX
- End User License Agreement