
Handbook of Fluoropolymer Science and Technology
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Handbook of Fluoropolymer Science and Technology
About this book
A comprehensive handbook on fluoropolymer synthesis, characterization, and processing
Fluoropolymers, one of the more durable classes of polymer materials, are known to enable novel technologies as a result of their remarkable properties. As key components in industry applications, fluoropolymers have established commercial interest and scientists have discovered more efficient approaches of handling them. This book reviews up-to-date fluoropolymer platforms as well as recently discovered methods for the preparation of fluorinated materials. It focuses on synthesis, characterization, and processing aspects, providing guidelines for practicing scientists and engineers. In addition, the book covers:
- Concepts and studies from leading international laboratories, including academia, government, and industrial institutions
- Emerging technologies and applications in energy, optics, space exploration, fuel cells, microelectronics, gas separation membranes, biomedical instrumentation, and more
- Current environmental concerns associated with fluoropolymers, relevant regulations, and growth opportunities
Overall, the chapters provide coverage of chemical methods and help the reader further understand how fluoropolymer research provides solutions for material challenges. The concepts in this book also inspire professionals to identify new markets and funding sources for fluoropolymer research and development.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
FLUORINATED POLYPHOSPHAZENES
1.1 BACKGROUND
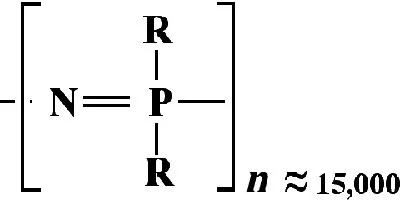
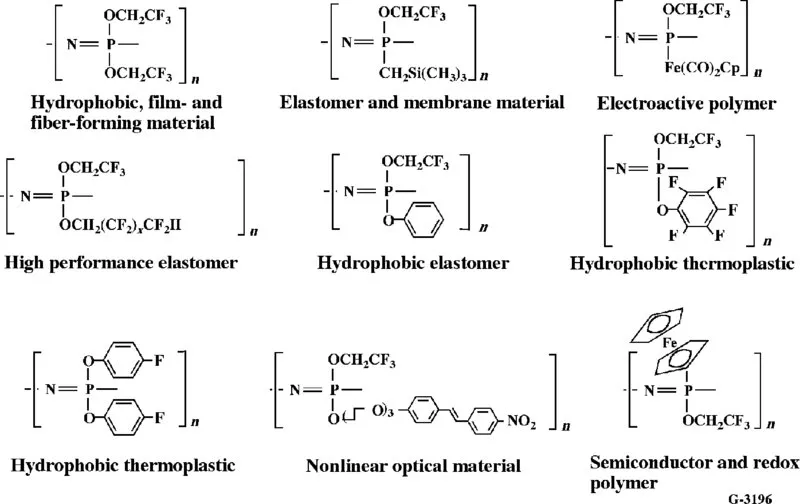
1.2 SYNTHESIS METHODS AND PROPERTY DEVELOPMENT

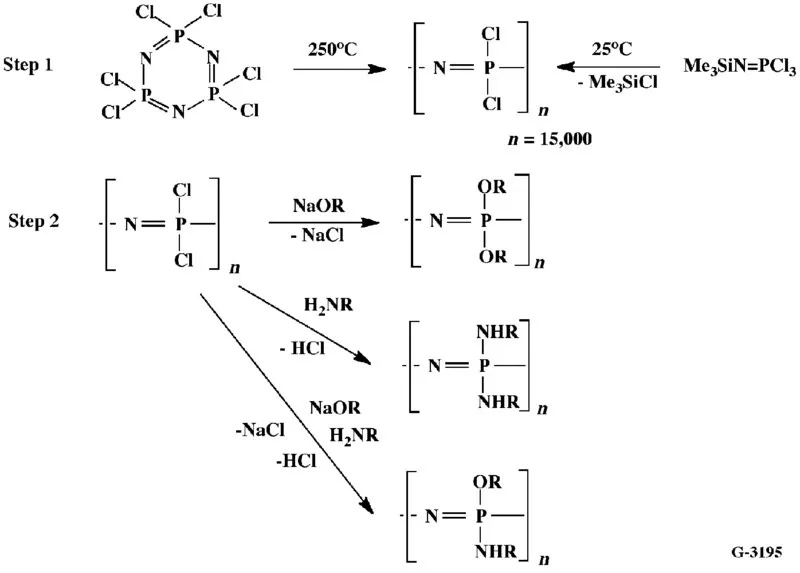
Table of contents
- Cover
- Titlepage
- Copyright
- Foreword
- In Memoriam
- Preface
- Contributors
- About the Editors
- 1 Fluorinated Polyphosphazenes
- 2 Mn2(CO)10-Visible Light Photomediated, Controlled Radical Polymerization of Main Chain Fluorinated Monomers and Synthesis of Block Copolymers Thereof
- 3 Interfacial Response of Semifluorinated Multi-Block Copolymers
- 4 Fluoropolymer Nanocomposites
- 5 Thermal Degradation and Pyrolysis of Polytetrafluoroethylene
- 6 Molecular Simulation of Fluoropolymers
- 7 Vapor Deposition of Fluoropolymer Surfaces
- 8 Functionalized and Functionalizable Fluoropolymer Membranes
- 9 Poly[Methyl(3,3,3-Trifluoropropyl)Siloxane]
- 10 Functional Fluorous Copolyoxetane Polymer Surface Modifiers
- 11 Self-Organizing Semifluorinated Methacrylate Copolymers
- 12 Synthesis of Fluoropolymers Using Borane-Mediated Control Radical Polymerization for Energy Storage Applications
- 13 Fluoropolymers in Supercritical Carbon Dioxide: Phase Behavior, Self-Assembly, and Stabilization of Water/Co2 Emulsions
- 14 Semifluorinated Polymers from Trifluorovinyl Aromatic Ether Monomers
- 15 Combustion Characterization of Energetic Fluoropolymer Composites
- 16 Amorphous Perfluoropolymers
- 17 Fluoropolymers for Sustainable Energy
- 18 Evolution of Academic Barricades for the use of Tetrafluoroethylene (TFE) in the Preparation of Fluoropolymers
- 19 Fluoropolymer Surfaces/Interfaces
- 20 Fluoropolymer Dielectrics
- 21 Fluoropolymers—Environmental Aspects
- 22 Fluorinated Ionomers and Ionomer Membranes Containing the bis[(perfluoroalkyl) sulfonyl]imide Protogenic Group
- 23 Fluorinated Silsesquioxanes
- 24 Multidimensional NMR of Fluoropolymers
- 25 Melt Processible Perfluoroplastics for Demanding Applications
- Index
- End User License Agreement