
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
The first book of its kind to focus on the chemistry of this promising class of molecules.
Edited by an innovator in the field, who has gathered an international team of well-established experts, this is a comprehensive overview of the rapidly developing field of polycyclic (hetero)arenes, specifically highlighting on their molecular design and the latest synthetic procedures, as well as chemical and physical properties. Each chapter is dedicated to a specific compound class, the first eight covering polycyclic arenes, including both planar and non-planar conjugated molecules, while chapters nine to twelve deal with polycylic heteroarenes according to the heteroatoms, namely N, B, S and P. Important current and emergent applications in the field are also discussed, ranging from molecular sensors to electronic devices.
The result is an essential reference for researchers in synthetic and physical organic chemistry, supramolecular chemistry, and materials science.
Edited by an innovator in the field, who has gathered an international team of well-established experts, this is a comprehensive overview of the rapidly developing field of polycyclic (hetero)arenes, specifically highlighting on their molecular design and the latest synthetic procedures, as well as chemical and physical properties. Each chapter is dedicated to a specific compound class, the first eight covering polycyclic arenes, including both planar and non-planar conjugated molecules, while chapters nine to twelve deal with polycylic heteroarenes according to the heteroatoms, namely N, B, S and P. Important current and emergent applications in the field are also discussed, ranging from molecular sensors to electronic devices.
The result is an essential reference for researchers in synthetic and physical organic chemistry, supramolecular chemistry, and materials science.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Polycyclic Arenes and Heteroarenes by Qian Miao in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Part I
Polycyclic Arenes
Chapter 1
Open-Shell Benzenoid Polycyclic Hydrocarbons
Soumyajit Das and Jishan Wu
1.1 Introduction
Graphene has been found to be an attractive material since its discovery in 2004 and has drawn enormous interest among researchers owing to its intrinsic electronic and magnetic properties. An indefinitely large graphene sheet, if cut along the two edge directions, can generate two distinct, well-defined small graphene nanoribbons (GNRs), which may be classified into “zigzag” edge (1, trans-polyacetylene) and “armchair” edge (2, cis-polyacetylene) structures (Figure 1.1). If graphene is cut in an all-zigzag fashion or in a triangular shape, the smallest unit that would come out is a phenalene unit (3) with one unpaired electron. The larger frameworks when cut in a zigzag shape will indefinitely lead to a series of non-Kekulé (i.e., open-shell) polycyclic hydrocarbons (PHs, 4–6) possessing one or more unpaired electrons, which can be termed as “open-shell graphene fragments” and are very interesting in terms of academic research. The number of unpaired electrons, or radicals, increases with the size of the framework, starting from phenalenyl monoradical to high-spin polyradical systems (3–6, Figure 1.1). If a graphene sheet is cut in an all-armchair fashion, it would lead to “all-benzenoid PHs,” as their structures can be represented as fully aromatic sextet rings (the six-membered rings highlighted in blue background) without additional double bonds (7). Even though the PHs of such category are larger in size with extended conjugation, they generally show high stability due to the stabilization through the existence of more number of Clar's aromatic sextets (8). The studies on hexa-peri-hexabenzocoronene and other extended all-benzenoid PHs have provided information on armchair-edged GNRs at the molecular level [1].
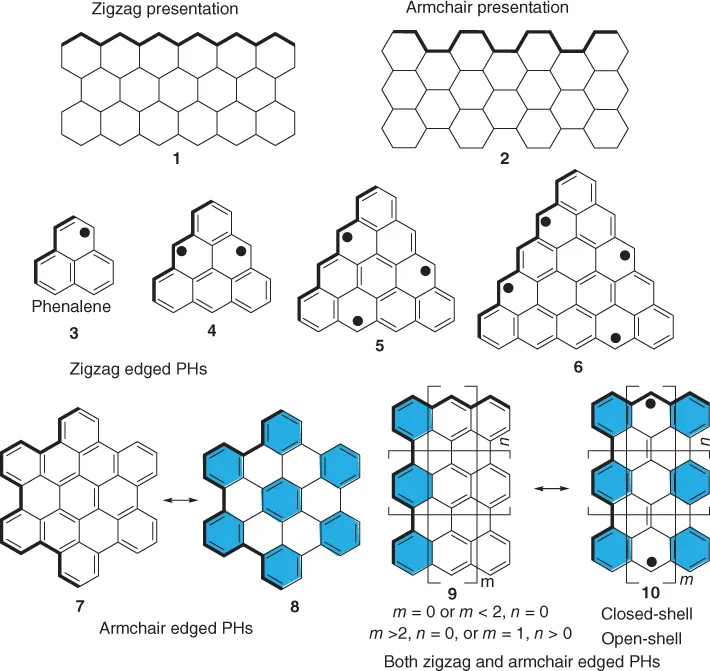
Figure 1.1 Molecular graphenes by fusion of benzene rings in different modes.
Another intriguing class of PHs would be rectangular-shaped GNRs, which are characterized by existence of both zigzag and armchair edges (9, Figure 1.1) with a typical Kekulé (i.e., closed-shell) structure. Their structures can be formulated by cycles in a monocyclic system symbolizing benzenoid aromatic sextet rings according to Clar's aromatic sextet rule [2]. Therefore, the more Clar's sextets the molecule can have, the more stable the system will be. Interestingly, recent theoretical and experimental work indicate that for rectangular PHs with extended zigzag edges, such as anthenes and periacenes, a remarkable open-shell diradical character will emerge when the conjugation is extended to a certain point (n > 0 for anthenes and m > 2 for periacenes), which originates from a narrowed bandgap and stabilization through more Clar's sextet rings in the diradical form (10, Figure 1.1). The molecule possesses more aromatic sextet rings in the diradical or poly-radical resonance forms and, if the recovered resonance energy can compensate the energy required to break a double bond, an open-shell singlet diradical or polyradical ground state could appear. Two very nice examples to validate such a statement are the teranthene and quarteranthene derivatives reported by Kubo et al., which will be discussed in following sections.
Most benzenoid PHs actually can be characterized by a closed-shell electronic configuration accommodating their π electrons only in bonding orbitals. However, researchers faced difficulties for certain types of PHs due to their high reactivity. An important work by Bendikov et al. came into spotlight in 2004 when their computational study on oligoacenes supported that the longer acenes possessed a nonzero bandgap with a singlet open-shell ground state followed by a higher energy triplet state [3]. The open-shell electronic configuration refers to the existence of one or more unpaired electrons, or radicals, in the molecular structure [4]. The electronic states of the open-shell systems with two unpaired electrons can be further divided into open-shell singlet, when the unpaired electrons adopt antiparallel spin, or open-shell triplet, when the unpaired electrons adopt parallel spin. Among all of the electronic states, the one with the lowest energy defines the ground state of π-conjugated systems. The high reactivity derived from open-shell nature of these systems in the ground state largely impedes their synthesis and isolation, but still continuous efforts have been made to synthesize and stabilize them due to the passion to understand the interplay of the unpaired electrons and delocalized π-electron systems and charge fluctuation, as well as the possibility of using them as molecule-based functional materials [5]. Therefore, the ground state can now be systematically studied by various spectroscopic te...
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- Table of Contents
- Preface
- List of Contributors
- Part I: Polycyclic Arenes
- Part II: Polycyclic Heteroarenes
- Index
- End User License Agreement