
Dynamic Modeling and Predictive Control in Solid Oxide Fuel Cells
First Principle and Data-based Approaches
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Dynamic Modeling and Predictive Control in Solid Oxide Fuel Cells
First Principle and Data-based Approaches
About this book
The high temperature solid oxide fuel cell (SOFC) is identified as one of the leading fuel cell technology contenders to capture the energy market in years to come. However, in order to operate as an efficient energy generating system, the SOFC requires an appropriate control system which in turn requires a detailed modelling of process dynamics.
Introducting state-of-the-art dynamic modelling, estimation, and control of SOFC systems, this book presents original modelling methods and brand new results as developed by the authors. With comprehensive coverage and bringing together many aspects of SOFC technology, it considers dynamic modelling through first-principles and data-based approaches, and considers all aspects of control, including modelling, system identification, state estimation, conventional and advanced control.
Key features:
- Discusses both planar and tubular SOFC, and detailed and simplified dynamic modelling for SOFC
- Systematically describes single model and distributed models from cell level to system level
- Provides parameters for all models developed for easy reference and reproducing of the results
- All theories are illustrated through vivid fuel cell application examples, such as state-of-the-art unscented Kalman filter, model predictive control, and system identification techniques to SOFC systems
The tutorial approach makes it perfect for learning the fundamentals of chemical engineering, system identification, state estimation and process control. It is suitable for graduate students in chemical, mechanical, power, and electrical engineering, especially those in process control, process systems engineering, control systems, or fuel cells. It will also aid researchers who need a reminder of the basics as well as an overview of current techniques in the dynamic modelling and control of SOFC.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1.1 Overview of Fuel Cell Technology
1.1.1 Types of Fuel Cells
- Solid Oxide Fuel Cell (SOFC): Solid oxide fuel cell uses a solid ceramic type oxide, and thus receives the name. Y2O3 stabilised ZrO2 (YSZ) is a common electrolyte used in SOFCs. The operating temperature of the fuel cell is usually high (600–1000 °C). Owing to the solid nature of the electrolyte and electrodes, the SOFC can be designed and fabricated in the most versatile ways, including planar and tubular designs.
- Molten Carbonate Fuel Cell (MCFC): Molten carbonate fuel cells use different combinations of alkali carbonates as an electrolyte. These carbonates are usually contained in a ceramic matrix. The operating temperature of MCFCs is also high, usually between 600 and 700 °C.
- Proton Exchange Membrane Fuel Cell (PEMFC): In this type of fuel cell, a polymeric ion exchange membrane is used as an electrolyte. The operating temperature of these cells is usually low (40–80 °C).
- Phosphoric Acid Fuel Cell (PAFC): The electrolyte in the PAFC is 100% phosphoric acid, which is held in a silicon carbide structure. The operating temperature of the fuel cell is about 150–220 °C, which is one of the attractive features of PAFC. This operating temperature makes it flexible to design the fuel cell and the balance of plant (BOP).
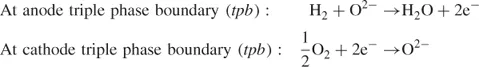
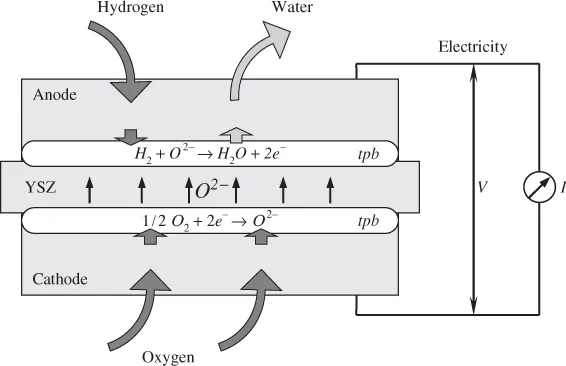
1.1.2 Planar and Tubular Designs
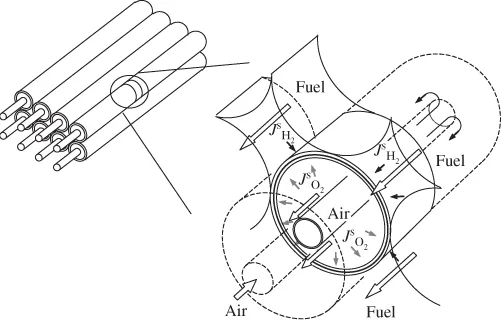
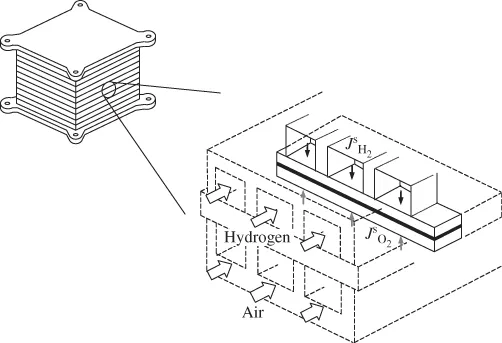
1.1.3 Fuel Cell Systems
1.1.4 Pros and Cons of Fuel Cells
- Unlike turbines, a fuel cell system does not have any moving components, and thus does not have any mechanical friction loss associated with it. It also provides a quiet operation and less maintenance.
- Unlike a heat engine, a fuel cell converts chemical energy directly into electrical energy. Thus, it is not limited by Carnot cycle efficiency.
- The exhaust (unreacted fuel) gas from the fuel cell can be used to generate excess power by coupling with a heat engine, thereby, increasing the efficiency.
- The efficiency of a fuel cell is not limited by size. Thus, a small fuel cell powering a laptop or a personal electronic gadget can generate power at the same efficiency as a 10 MW fuel cell power plant.
- A wide range of fuels may be used for fuel cells.
- As the reaction inside a fuel cell occurs between specific ions only, it limits the release of NOx and SOx to the environment.
- Fuel cells are expensive compared to other energy producing technologies at least at the moment.
- Most fuel cells use hydrogen as fuel, and it impedes commercialisation of these devices because of the cost and complexities associated with the production, storage and transportation of hydrogen.
- In comparison with batteries, fuel cells have lower power densities and shorter lifetimes.
- Impurity of fuel gas may poison catalysts in electrodes.
1.2 Modelling, State Estimation and Control
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- Acknowledgments
- List of Figures
- List of Tables
- Chapter 1: Introduction
- Part One: Fundamentals
- Part Two: Tubular SOFC
- Part Three: Planar SOFC
- Appendix A: Properties and Parameters
- References
- Index