
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
The Toxicology of Methanol
About this book
The Toxicology of Methanol presents a single source of information and an understanding of the toxicity of methanol from animal data, potential environmental effects as well as human effects. The animal data, which goes to making up the majority of the data on the toxicity of methanol and the mechanism of action, is reviewed as it relates to the potential toxicity in humans.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access The Toxicology of Methanol by John J. Clary in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
Methanol Production and Markets: Past, Present, and Future
Methanol has a long proud history dating back to ancient times when Egyptians formed it as a byproduct of charcoal fabrication from wood (Crocco, 2002), which was then used to preserve mummies. Not much changed in the intervening centuries to improve the process. In 1923, world methanol production stood at just 30,000 tons (1 ton of methanol contains 333 gallons), distilled from 3 million tons of wood feedstock. In the same year, Matthias Pier of BASF produced the first railcar load of synthetic methanol from a converted ammonia loop. In post-World War II Germany, methanol was produced from petroleum liquids and coal for fuel use. In the 1960s and 1970s, companies such as ICI in the United Kingdom and Lurgi in Germany began developing specialized catalysts for methanol synthesis from natural gas in low-pressure processes. Over the next two decades, the methanol industry would grow from a “captive” market with plants located next to their downstream derivative (i.e., formaldehyde or acetic acid typically) to a global “merchant” market, with methanol widely exported around the world.
In 2011, world methanol demand topped 45 million tons (CMAI, 2011) and with 65% of this consumption being traded from one continent to another, methanol is clearly one of the world's most widely distributed chemical commodities. Owing to the steady growth of methanol demand, we have seen a significant rebalancing of methanol production. Referred to in the industry as a “rationalization,” the plants in regions with rapidly increasing natural gas feedstock costs have been closed, as new “mega” methanol plants are built in countries where natural gas is more plentiful and less expensive. These “mega” methanol plants have capacities of 5000 tons per day (600 million gallons per year), with a single plant representing close to 5% of global production. Production capacity in North America and Western Europe fell from 13.3 million tons in 1999 to just 900,000 tons in 2010. During this same time period, production capacity jumped from 13.1 million tons to over 24.5 million tons in South America (led by Trinidad and Tobago) and the Middle East. The real wild card in the global methanol industry is China, which saw the production capacity soaring from just 1.2 million tons in 1999 to 40 million tons by 2011. By 2007, China had become the world's largest methanol producer and consumer, with the breakneck pace of new methanol plant construction building further momentum for growth.
Today, we are seeing the pendulum beginning to swing back again. We are now seeing a re-emergence of North American methanol capacity, driven by the increasing availability of shale gas and its impact on pushing natural gas prices below $4 per MMBtu. Formerly, mothballed methanol plants in Canada and Texas have been restarted, with one of these facilities recouping its restart costs in just 7 months. One major producer is looking to ship one or two methanol plants from South America, which have had challenges accessing natural gas, to the U.S. gulf coast, which now boasts the lowest costs for available natural gas in the world market.
Today, most of the world's methanol production comes from the steam reformation of natural gas, characterized by the two-step equation:
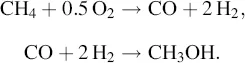
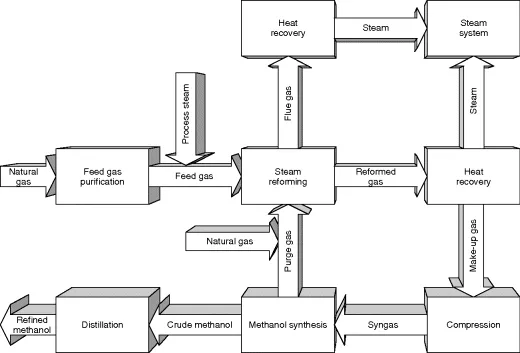
The methanol production process involves four basis steps (Figure 1.1): (1) feed gas purification to remove natural gas components such as sulfur that can poison catalysts; (2) steam reforming to saturate the hydrocarbons producing a synthesis gas of carbon dioxide and hydrogen; (3) methanol synthesis by passing the synthesis gas over a catalyst bed at high temperatures and pressures to produce crude liquid methanol; and (4) distillation typically accomplished in a two-step process to remove water and some ethanol created in the process. The finished methanol must meet rigorous purity standards generally in the order of 99.85% (ASTM D-1152/97).
Figure 1.1 Conventional methanol production.
The production of methanol from natural gas, coal, or biomass shares a number of basic processing steps (Zuberbühler, 2005). The feedstock must be gasified by heating in the presence of little or no oxygen to produce a synthesis gas made up of carbon monoxide, hydrogen, carbon dioxide, and water (along with various other gases). This “syngas” is then catalytically processed into liquid methanol while much of the “equipment” for gasification involves mature technologies using recognized feedstocks. While majority of methanol is produced through the steam reformation of natural gas, China has focused on converting its vast coal resources to methanol via gasification. For “biomethanol,” the immature part of the equation is the first step, the gasification of biomass (a feedstock with different characteristics). Once the syngas is generated, we know what to do; it is to get to that point using biomass feedstocks that have received little attention. In the global push to ferment plant starches to ethanol, little work has been done on biomass gasification to methanol, the other alcohol.
When using biomass as a feedstock for biofuel production, there are four basic production pathways: (1) biochemical conversion using enzymes and microorganisms to breakdown biomass into sugars used for fuel production; (2) thermochemical conversion employing heat energy and chemical catalysts to convert biomass into fuels; (3) gasification to dissociate biomass in a high-temperature, oxygen-starved environment to produce synthesis gas; and (4) pyrolysis using high temperatures in an oxygen-free environment to encourage the decomposition of biomass. As the simplest alcohol, methanol can be produced from virtually any organic material using some form of these processes. However, the most common methods employed to produce methanol from biomass involve the gasification of “dry biomass” (forest thinnings, waste wood, pulp mill byproducts, municipal solid waste) and the fermentation of “wet” biomass (animal manure, wastewater, industrial wastewater, algae, seaweed) typically through anaerobic digestion (Specht and Bandi).
Biomass gasification for methanol production is especially attractive as high carbon conversion rates and fuel yields mean that the biomass resource can be completely utilized. By comparison, conventional production processes for the biochemical conversion of plant starch and oil plants use only a small fraction of the biomass feedstock. For example, it is understood that production of ethanol from corn yields 7.2 dry tons/ha/year, or 76 GJ/ha/year, whereas the production of methanol from wood yields 15 dry tons/ha/year or the equivalent of 177 GJ/ha/year (Williams et al., 1995). In other words, through gasification, 1 ton of woody biomass can produce 165 gallons of methanol while the hoped for yields for cellulosic ethanol is targeted to around 60–70 gallons of fuel per ton of biomass. As the Swedish Minister for Enterprise and Energy Deputy Prime Minister Maud Olofsson put it, “We need to move away from first generation in ethanol manufacturing and further to second and third generation, which is all about cellulose material and gasification, and this implies therefore room for methanol and synthetic diesel” (Olofsson, 2012).
Further, the production of methanol from biomass gasification may turn out to be an evolutionary stepping-stone to a “fourth” generation technology. In his seminal text “Beyond Oil and Gas: The Methanol Economy” (Olah, 2006), the Nobel Prize Laureate Dr George A. Olah of the University of Southern California argues that we may soon recycle atmospheric carbon dioxide using catalytic and electrochemical methods to produce liquid methanol. As Dr. Olah states, “It should be emphasized that the ‘Methanol Economy’ is not producing energy. In the form of liquid methanol, it only stores energy more conveniently and safely compared to extremely difficult to handle and highly volatile hydrogen gas, the basis of the ‘hydrogen economy’. Besides being a most convenient energy storage material and a suitable transportation fuel, methanol can also be catalytically converted to ethylene and/or propylene, the building blocks of synthetic hydrocarbons and their products, which are currently obtained from our diminishing oil and gas resources.”
This is an important point, as the petrochemical industry has grown hand in hand with the petroleum industry for good and bad. Methanol is a basic building block for hundreds of chemical commodities such as formaldehyde and acetic acid used in products ranging from building materials and plastics to paints, adhesives, and solvents. We even color methanol blue for the windshield wash fluid in your car today.
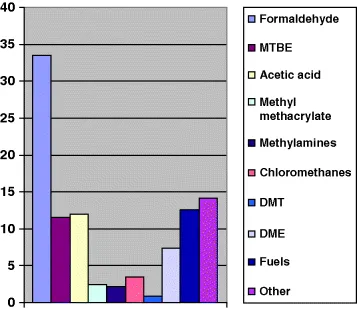
As a chemical building block, methanol is a key component of hundreds of products that touch our daily lives. The largest global market for methanol is as a feedstock for the production of formaldehyde. Engineered woods used in building our homes and furniture are bonded with resins produced from formaldehyde. In our cars, urethanes and plastics used in essential components also contain formaldehyde. Methanol is also used in the production of acetic acid, which then is used for making polyethylene teraphthalate (PET) plastic used in beverage packaging. Acetic acid is the basic component of terapthalic acid (PTA), which is used in making polyester fiber for our clothing and carpets. Vinyl acetage monomer (VAM) is also produced for acetic acid and is used in the manufacture of water-based paints and adhesives. On a global basis, the fuel additive methyl tertiary butyl ether (MTBE) is still used to increase octane performance and reduce emissions in vehicles. MTBE is produced from methanol and butanes and continues to play an important role as a fuel oxygenate in Asia and the Middle East.
As can be seen in Figure 1.2, methanol is both an important chemical commodity and an energy fuel. The “Other” category includes several applications for consumer products that are widely familiar, including windshield wash fluid and “sterno” cooking fuels. While the growth of methanol's chemical markets is generally on pace with other chemicals at about 3–5% per year, markets for methanol fuels are expanding at a robust 25–40% per annum. In terms of consumer exposure to methanol, the use of methanol transportation fuels—primarily in the act of vehicle refueling—represents the largest potential exposure route. However, the 5-minutes-exposure to the inhalation of methanol vapors from refueling a methanol compatible vehicle is expected to generate less of a methanol uptake than drinking a can of diet soda containing the sweetener aspartame (which metabolizes in the body to methanol).
Figure 1.2 Global methanol consumption.
In the United States, there are now more than 8 million ethanol flexible-fuel vehicles (FFV) on the road although only a few alternative fuel vehicle buffs will recall that FFV technology began with 20,000 methanol/gasoline cars sold in the 1980s and 1990s. The Renewable Fuel Standard, established by the U.S. Congress in 2007, calls for the use of 36 billion gallons of renewable fuels by 2022, which many translate into a mandate for corn ethanol and cellulosic ethanol. Actually, methanol produced from renewable biomass feedstocks will count too, and may make more sense (and cents).
The State of California often seems like the conscious of the global automotive industry, pushing for the market introduction of more efficient and cleaner vehicle technologies. We can trace this history back to the late 1970s when the California Energy Commission began testing dedicated methanol-fueled vehicles. Operating vehicles on neat methanol had its benefits and drawbacks. These dedicated vehicles would take advantage of methanol's higher octane content (100 octane for methanol versus 87–94 for gasoline) by using higher compression ratios to increase fuel efficiency and dramatically reduce emissions. There were problems with cold-starting vehicle on neat methanol and concerns with the visibility of methanol flames in bright, sunlight conditions. By the early 1980s, the effort turned to methanol FFVs capable of running on a blend of up to 85% methanol and 15% gasoline (called M-85) in the same fuel tank. The use of M-85 assisted with cold starting and imparted visibility to methanol flames. The real drive behind FFV technology was to help overcome the problem of limited availability of methanol fueling stations in the early years of the program. The objective was to introduce large numbers of methanol FFVs, build a broad fueling infrastructure network, then transition back to dedicated methanol vehicles.
With encouragement from the state, a series of initiatives led to the demonstration of 18 different models of methanol-fueled cars from a dozen automakers. The state also established a methanol fuel reserve and entered into 10-year leases with gasoline retailers for the establishment of a network of 60 public retail methanol-fueling pumps and 45 private fleet-accessible fueling facilities. Over 15,000 methanol FFVs would find a home on California's streets and freeways, along with hundreds of methanol-fueled transit and school buses. During the peak of the program in 1993, over 12 million gallons of methanol was used as a transportation fuel in the state. Through these efforts, FFVs were developed as a largely inexpensive “off-the-shelf” technology, and the challenges of dispensing alcohol fuels were solved. In addition, fearing the potential market share loss from growing methanol fuel use, the major oil companies began introducing cleaner “reformulated” gasolines that eroded many of the clean air benefits of using methanol.
Ultimately, only four methanol FFV models moved from prototype demonstration to commercial availability (Ford Taurus 1993–1998 model years; Chrysler Dodge Spirit/Plymouth Acclaim 1993–1994 model years; Chrysler Concorde/Intrepid 1994–1995 model years; and the General Motors Lumina 1991–1993 model years). By the mid-1990s, automakers had already abandoned further development work on methanol, turning instead to work on compressed natural gas and battery electrics. Today, China has picked up the methanol torch, with over 2.3 billion gallons methanol blended in gasoline (M-15, M-30, M-85, and M-100) in 2011 for use in passenger cars, taxis, and bus fleets. Chinese automakers are introducing new models of methanol FFVs, while national fuel standards for methanol fuel blending have been adopted to grow the market in an organized manner. China now views coal-based methanol as a strategic transportation fuel.
This in an important point as the use of methanol as a transportation fuel offers a viable means of transitioning from fossil-based fuels to renewable fuels. Liquid secondary energy carriers have a much bigger market potential than gaseous hydrogen (or liquid hydrogen, t, −253°C) (30). Methanol can be p...
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- Contributors
- Chapter 1: Methanol Production and Markets: Past, Present, and Future
- Chapter 2: Methanol: Fate and Transport in the Environment
- Chapter 3: Human Toxicity
- Chapter 4: General Animal and Aquatic Toxicity
- Chapter 5: Developmental and Reproductive Toxicology of Methanol
- Chapter 6: Exploring Differences Between PBPK Models of Methanol Disposition in Mice and Humans: Important Lessons Learned
- Chapter 7: Oxidative Stress and Species Differences in the Metabolism, Developmental Toxicity, and Carcinogenic Potential of Methanol and Ethanol
- Chapter 8: Methanol and Cancer
- Index