![]()
Chapter 1
Principles of Combustion
1.1 Introduction
Flaring is defined as the controlled burning of off gases in the course of routine oil and gas or chemical manufacturing operations. This burning or combustion is accomplished at the end of a flare stack or boom.
Combustion is often described as a simple chemical reaction in which oxygen from the atmosphere reacts rapidly with a substance, generating heat. But it is in fact a very complex series of chemical reactions. The most common organic compounds are hydrocarbons, which are composed of carbon and hydrogen. The simplest hydrocarbon is methane, each molecule of which consists of one carbon atom and four hydrogen atoms. It is the first compound in the family known as alkanes. The physical properties of alkanes change with increasing number of carbon atoms in the molecule, those with one to four being gases, those with five to ten being volatile liquids, those with 11 to 18 being heavier fuel oils and those with 19 to 40 being lubricating oils. Longer carbon chain hydrocarbons are tars and waxes. The first ten alkanes are:
- CH4 methane (gas)
- C6H14 hexane (liquid)
- C2H6 ethane (gas)
- C7H16 heptane (liquid)
- C3H8 propane (gas)
- C8H18 octane (liquid)
- C4H10 butane (gas)
- C9H20 nonane (liquid)
- C5H12 pentane (liquid)
- C10H22 decane (liquid)
Alkenes are similar but their molecular structure includes double bonds (examples are ethylene and propylene). Alkynes contain triple bonds (example is acetylene). The above compounds are all known as aliphatics. Aromatic hydrocarbons such as benzene have a ring molecular structure and burn with a smoky flame.
When hydrocarbons burn they react with oxygen, producing carbon dioxide and water (although if the combustion is incomplete because there is insufficient oxygen, carbon monoxide will also form).
More complex organic compounds contain elements such as oxygen, nitrogen, sulfur, chlorine, bromine, or fluorine, and if these burn, the products of combustion will include other compounds as well. For example, substances containing sulfur such as oil or coal will result in sulfur dioxide whilst those containing chlorine such as methyl chloride or polyvinyl chloride (PVC) will result in hydrogen chloride.
This chapter focuses on combustion principles which are essential to the selection and safe operation of flares. Without a fundamental understanding of combustion principles, the proper selection of and safe operation of flares are not possible. Note also that Appendix A contains various physical and thermodynamic properties data for gases. The information has been assembled for the more knowledgeable reader to aid in any preliminary calculations for estimating flare sizes, specifying flow conditions, and determining flammability.
1.2 Combustion Basics
Combustion is a chemical reaction, and specifically it is an oxidation reaction. Oxidation is defined as the chemical combination of oxygen with any substance. In other words, whenever oxygen (and some other materials) combines chemically with a substance, that substance is said to have been oxidized. Rust is an example of oxidized iron. In this case the chemical reaction is very slow. The very rapid oxidation of a substance is called combustion.
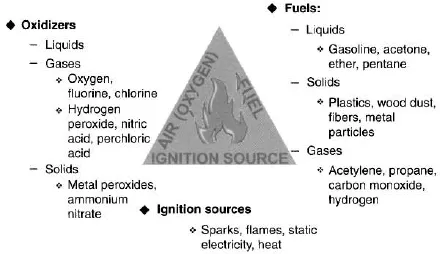
There are three basic explanations that are used to describe the reaction known as combustion. They are the fire triangle, the tetrahedron of fire, and the life cycle of fire. Of the three, the first is the oldest and best known, the second is accepted as more fully explaining the chemistry of combustion, while the third is a more detailed version of the fire triangle.
The fire triangle explanation is simplistic, but provides a basic understanding of the three entities that are necessary for a fire to occur. This theory states that there are three things necessary to support combustion:
- fuel;
- oxygen (or an oxidizer); and
- heat (or energy).
These three components can be represented as the three sides of a triangle, stating that as long as the triangle is not complete, that is, the legs are not touching each other to form the closed or completed triangle, combustion cannot take place. See Figure 1.1.
The theory or explanation, as stated, is correct. Without fuel to burn, there can be no fire. If there is no oxygen present, there can be no fire (technically, this is not correct, but we can make the fire triangle theory technically correct by changing the oxygen leg to an oxidizer leg). Finally, without heat or a source of energy, there can be no fire. This last statement must also be brought up to date. The fact is that heat is just one form of energy: it is really energy that is necessary to start a fire. This difference is mentioned because there are some instances where light or some other form of energy may be what is needed to start the combustion reaction. It is best to change the “heat” leg of the fire triangle to the “energy” leg. Therefore, our fire triangle has three sides representing fuel, oxidizer, and energy.
A fuel is anything that will burn. Fuels may be categorized into the following classes:
- Elements (which include the metals, and some nonmetals such as carbon, sulfur, and phosphorus);
- Hydrocarbons;
- Carbohydrates (including mixtures that are made up partially of cellulose, like wood and paper);
- Many covalently bonded gases (including carbon monoxide, ammonia, and hydrogen cyanide); and
- All other organically based compounds.
We are only concerned with gaseous and vapor streams that include hydrocarbons, covalently bonded gases, and of course organically based waste gas streams when it comes to flaring operations.
The list of materials that will combust is quite long, and one must not forget that the list includes not only the pure substances such as the elements and compounds that make up the list, but mixtures of those elements and compounds. Examples of mixtures would include natural gas, which is a mixture of methane (principally), ethane, and a few other compounds, and gasoline, which is a mixture of the first six liquid alkanes (pentane, hexane, heptane, octane, nonane, and decane), plus a few other compounds.
The oxidizer leg of the triangle usually refers to air, since it is the most common oxidizing agent encountered and is readily available. Oxygen does not burn. It is consumed during combustion.
The third leg of the fire triangle, the energy leg, provides the source of energy needed to start the combustion process. This energy can be provided in one or more of several ways. The energy can be generated chemically by the combustion of some other fuel, or it can be generated by some other exothermic chemical reaction. An exothermic reaction is defined as the emission or liberation of heat (or energy). This is the opposite of endothermic, which is defined as the taking-in or absorption of heat (or energy).
Energy may also be generated by mechanical action, that is, the application of physical force by one body upon another. Examples of this are the energy created by the friction of one matter upon another or the compression of a gas. The force of friction in one case may produce energy that manifests itself as heat, while friction in the other case may result in a discharge of static electricity. Static electricity is created whenever molecules move over and past other molecules. This happens whether the moving molecules are in the form of a gas, a liquid, or a solid. This is the reason why leaking natural gas under high pressure will ignite. This is also the reason why two containers must be bonded – connected by an electrical conductor – when you are pouring flammable liquids from one container to another. In any case, the amount of energy present and/or released could be more than enough to start the combustion reaction.
A third method of generation of energy is electrical, which is the preferred method of igniting flares. This method manifests itself as heat, produced from an electrical circuit in combination with a gas pilot.
The second popular explanation combustion is the tetrahedron theory. This theory encompasses the three concepts much like the fire triangle theory, but adds a fourth side to the triangle to make up a pyramid or tetrahedron. This fourth side is referred to as the chain reaction of combustion. The explanation states that when energy is applied to a fuel like a hydrocarbon, some of the carbon-to-carbon bonds break, leaving an unpaired electron attached to one of the molecular fragments caused by the cleavage of the bond, thus creating a free radical. This molecular fragment with the unpaired electron, or “dangling” bond, is highly reactive, and will therefore seek out some other material to react with in order to satisfy the octet rule. The same energy source that provided the necessary energy to break the carbon-to-carbon bond may have also broken some carbon-to-hydrogen bonds, creating more free radicals, and also broken some oxygen-to-oxygen bonds, creating oxide radicals. This mass breaking of bonds creates the free radicals in a particular space, and in a number large...