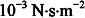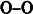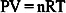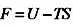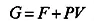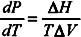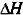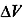![]()
Chapter 1
Water: A Molecule Endowed with Extraordinary Physicochemical Properties
1.1. Molecular geometry and electrical properties
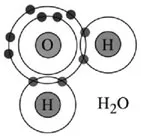
A water molecule consists of an oxygen atom bonded to two hydrogen atoms. In water, each hydrogen atom is bound to the oxygen by a pair of electrons. However, only two of the six outer-shell electrons of oxygen are used to form covalent bonds, the remaining four being organized into two non-bonding pairs (
Figure 1.1). The four electron pairs surrounding the oxygen tend to arrange themselves as far from each other as possible in order to minimize repulsions between these clouds of negative charge. However, the two non-bonding pairs exert a strong repulsion against the two covalent bonding pairs, which results in a deformed tetrahedral geometry with a
angle of 105° instead of the theoretical angle of 109°. As a result, the H
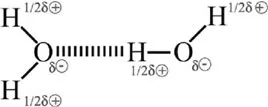
2O molecule is electrically neutral even though the electrical charges are not distributed uniformly. Indeed, a negative charge is associated with the oxygen atom while the hydrogen atom carries a positive charge (
Figure 1.2). This electronic configuration defines the polar structure of water molecules, which consequently have a mutual attraction and tend to stick together.
This process is called “hydrogen bonding” and explains why water is a liquid instead of a gas under standard conditions (close to the Earth’s surface pressure and temperature conditions). In comparison to a covalent bond, the hydrogen bond is so weak that the timescale of its life expectancy is in the order of the picosecond (10
−12 s), therefore explaining the low molecular viscosity of water (
at 20°C) compared to many other liquids at a given temperature. This low molecular viscosity plays a key role in the regulation of osmotic pressure in body fluids.
Figure 1.1. Bonding and non-bonding electronic pairs of the outer shell in the water molecule
Figure 1.2. The dipolar water molecules forming hydrogen bonding
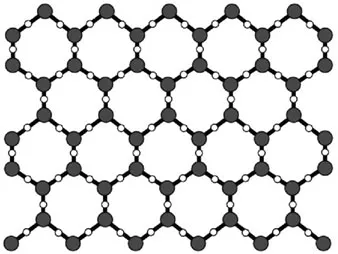
In ordinary ice, each water molecule forms four hydrogen bonds to the nearest oxygen neighbors with
distances of 2.76
(
Figure 1.3). The triple O angles are 109° according to a lattice structure with a tetrahedral coordination. This basic unit is repeated in three dimensions to build the ordinary ice crystals with hexagonal symmetry that can be observed in snowflakes.
Figure 1.3. A tetrahedral coordination and hexagonal symmetry of the crystal lattice of water ice
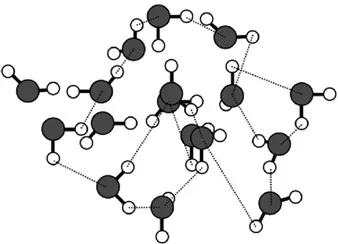
When ice starts melting and forms a thin layer of liquid water (Figure 1.4), the crystal lattice breaks down as thermal motions distort and finally break hydrogen bonds.
Figure 1.4. The “disordered” structure of the water molecule
1.2. Phase diagram
Phase diagrams define fields in temperature and pressure where a substance is characterized by the same chemical composition and physical state. The most popular equation of state was defined for an ideal gas during the second part of the 17th Century and is known as Boyle’s law or the Boyle–Mariotte law:
where P is the pressure, V is the volume, n is the number of moles, T is the absolute temperature and R is the universal gas constant.
In 1873, van der Waals determined the first equation of state able to predict the conditions of coexistence between vapor and liquid phases, which was further refined to give the Redlich–Kwong equation in 1949. More recently, the Helmholtz free energy function (F), applied to a pure substance such as water, was used to determine its thermodynamic properties, such as caloric properties, isochoric and isobaric heat capacity, speed of sound, and differences in enthalpy and internal energy. This work led to the definition of a fundamental equation of state that was proposed by the International Association for the Properties of Water and Steam in 1995 (IAPWS-95) (Figure 1.5).
The Helmholtz free energy function is defined as follows:
where U is the internal energy of the system, T is the absolute temperature and S is the entropy. It is noteworthy that F is related to the Gibbs free energy function as follows:
In phase diagrams, the physical states of a given substance are defined by fields limited by coexistence curves also called “binodal curves”. In pressure–temperature diagrams, the slopes of these curves can be calculated by using the Clausius–Clapeyron equation according to:
where
and
are, respectively, the enthalpy and specific volume changes that take place during the phase transition.
Figure 1.5. The phase–boundary curves of pure water computed after the equation of state developed according to IAPWS-95 [WAG 02]
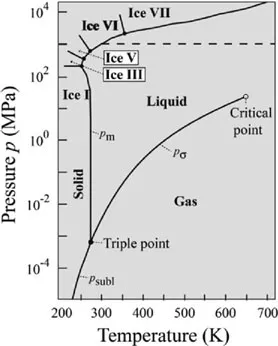
On the Earth, life emerged under P–T conditions close to the triple point of water (Figure 1.6), which means that the three phases (gas, liquid and solid) coexist, having identical Gibbs free energies. A “critical point” occurs at the end of a phase line where the properties of the two phases become indistinguishable from each other. Boundary crossings between the solid–liquid–gaseous fields of the phase water diagram correspond to specific processes in the physical state of the H2O molecule. For example, crossing the boundary from the solid to the gaseous state is called “sublimation”, while the reverse pathway is called “deposition”, the term “condensation” being reserved for the changing state from vapor to liquid water (Figure 1.6).
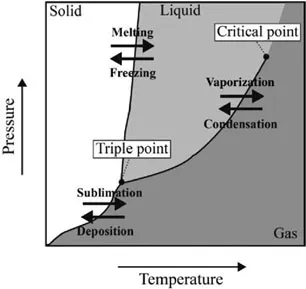
The known ices, 16 types so far defined, are distinguished on the basis of their structure (Figure 1.7). The low-pressure phases (hexagonal ice (Ih), cubic ice (Ic) and ice (XI)) are characterized by quite a perfect tetrahedral geometry built with the oxygen atoms, while some distortion of the crystal lattice affects the high-pressure polymorphs (ices II–IX and ices XII–XV).
Figure 1.6. Terms corresponding to phase changes around the triple point of water
Figure 1.7. Phase diagram of water with the stability regions for the 16 known polymorphs of ice [COG 11]
Hexagonal (d = 926 kg·m–3), cubic (d = 933 kg·m–3) ices and ice XI (d = 930 kg·m–3) are less dense than liquid water, whereas the other ices are all denser than liquid water with ...