Proteins
Biochemistry and Biotechnology
Gary Walsh
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
Proteins
Biochemistry and Biotechnology
Gary Walsh
Über dieses Buch
Proteins Biochemistry and Biotechnology 2e is a definitive source of information for all those interested in protein science, and particularly the commercial production and isolation of specific proteins, and their subsequent utilization for applied purposes in industry and medicine. Fully updated throughout with new or fundamentally revised sections on proteomics as, bioinformatics, protein glycosylation and engineering, well as sections detailing advances in upstream processing and newer protein applications such as enzyme-based biofuel production this new edition has an increased focus on biochemistry to ensure the balance between biochemisty and biotechnology, enhanced with numerous case studies. This second edition is an invaluable text for undergraduates of biochemistry and biotechnology but will also be relevant to students of microbiology, molecular biology, bioinformatics and any branch of the biomedical sciences who require a broad overview of the various medical, diagnostic and industrial uses of proteins. • Provides a comprehensive overview of all aspects of protein biochemisty and protein biotechnology
• Includes numerous case studies
• Increased focus on protein biochemistry to ensure balance between biochemisty and biotechnology
• Includes new section focusing on proteomics as well as sections detailing protein function and enzyme-based biofuel production "With the potential of a standard reference source on the topic, any molecular biotechnologist will profit greatly from having this excellent book. " ( Engineering in Life Sciences, 2004; Vol 5; No. 5) "Few texts would be considered competitors, and none compare favorably." ( Biochemistry and Molecular Education, July/August 2002) "...The book is well written, making it informative and easy to read..." ( The Biochemist, June 2002)
Häufig gestellte Fragen
Information
Chapter 1
Proteins and proteomics
1.1 Proteins, an introduction
- Primary structure: the specific amino acid sequence of its polypeptide chain(s), along with the exact positioning of any disulfide bonds present.
- Secondary structure: regular recurring arrangements of adjacent amino acid residues, often over relatively short contiguous sequences within the protein backbone. The common secondary structures are the α-helix and β-strands.
- Tertiary structure: the three-dimensional arrangement of all the atoms which contribute to the polypeptide. In other words, the overall three-dimensional structure (conformation) of a polypeptide chain, which usually contains several stretches of secondary structure interrupted by less ordered regions such as bends/loops.
- Quaternary structure: the overall spatial arrangement of polypeptide subunits within a protein composed of two or more polypeptides.
| Protein | Polypeptide chains | Total no. of amino acids | Molecular mass (Da) | Biological function |
| Insulin (human) | 2 | 51 | 5800 | Complex, but includes regulation of blood glucose levels |
| Lysozyme (egg) | 1 | 129 | 13,900 | Enzyme capable of degrading peptidoglycan in bacterial cell walls |
| Interleukin-2 (human) | 1 | 133 | 15,400 | T-lymphocyte-derived polypeptide that regulates many aspects of immunity |
| Erythropoietin (human) | 1 | 165 | 36,000 | Hormone which stimulates red blood cell production |
| Chymotrypsin (bovine) | 3 | 241 | 21,600 | Digestive proteolytic enzyme |
| Subtilisin (Bacillus amyloliquefaciens) | 1 | 274 | 27,500 | Bacterial proteolytic enzyme |
| Tumour necrosis factor (human TNF-α) | 3 | 471 | 52,000 | Mediator of inflammation and immunity |
| Haemoglobin (human) | 4 | 574 | 64,500 | Gas transport |
| Hexokinase (yeast) | 2 | 800 | 102,000 | Enzyme capable of phosphorylating selected monosaccharides |
| Glutamate dehydrogenase (bovine) | ~40 | ~8300 | ~1,000,000 | Enzyme that interconverts glutamate and α-ketoglutarate and NH4+ |
1.2 Genes, genomics and proteomics
- sequence the entire DNA complement of the cell; and
- to physically map the genome arrangement (assign exact positions in the genome to the various genes and non-coding regions).
- at any given time a proportion of genes are not being expressed;
- of those genes that are expressed, some are expressed at higher levels than others;
- the proteome is dynamic rather than static because the exact subset of proteins expressed (and the level at which they are expressed) in any cell changes with time in response to a myriad of environmental and genetic influences;
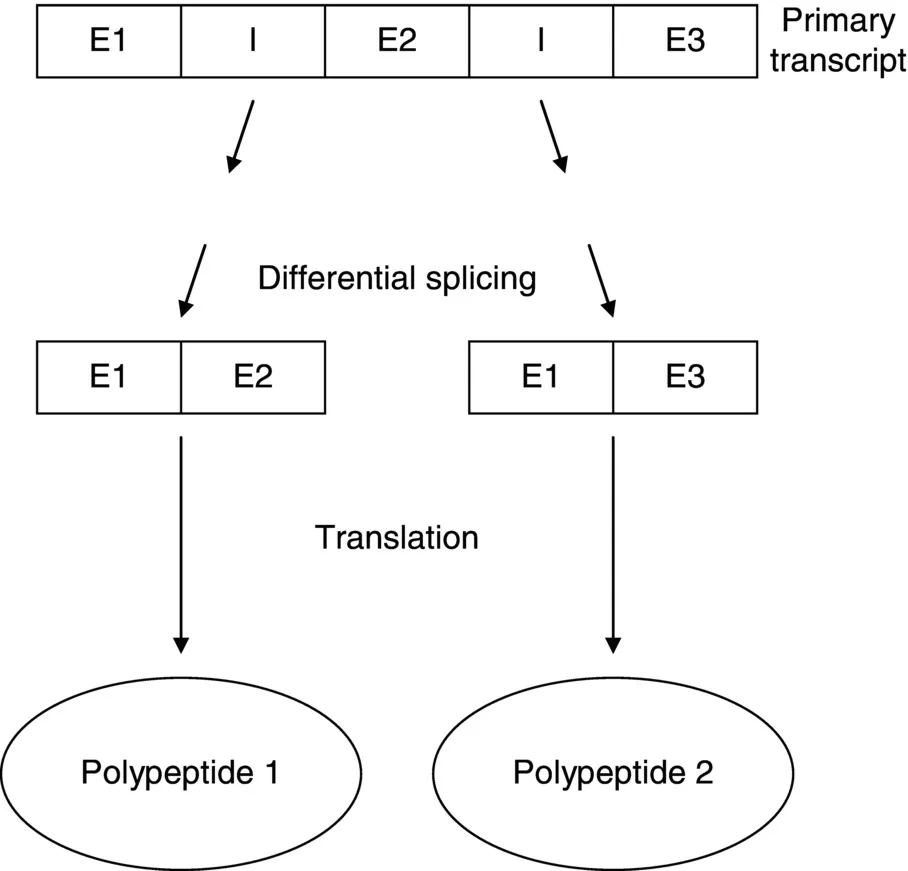
- for eukaryotes, a single gene can effectively encode more than one polypeptide if its mRNA undergoes differential splicing (Figure 1.1);
- many eukaryotic proteins undergo PTM.