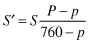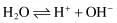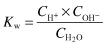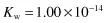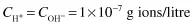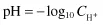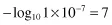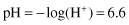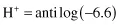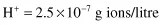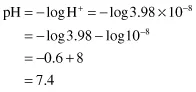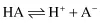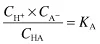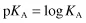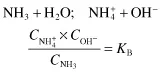![]()
1
The Aquatic Environment
INTRODUCTION
Disease, in fishes, is closely linked to environmental stress. In the wild, they generally have some degree of freedom to modify their environment. They can move to more suitable conditions if faced with a negative environmental change such as a reduction in oxygen level or increase in temperature. Infected fish will even move to a warmer area to create an enhanced body temperature as an aid to increasing the rate of inflammatory response. In culture conditions, on the other hand, they have limited opportunity to choose their external environmental conditions. It is thus important that the conditions under which they are held provide environmental parameters suitable for all of the requirements of the particular species.
The aquatic environment encompasses a wide variety of features, virtually all of which influence the maintenance of homeostasis, essential for growth and reproduction of fishes. If altered beyond acceptable limits, they may predispose to, or actually cause, a wide range of disease processes. Among the most important environmental parameters are physical factors such as the temperature, the intensity and periodicity of light (including shading and background hue), the chemical composition of the water and its biological content, the availability of space and food and the frequency of fright stimuli such as moving shadows (EFSA 2008). Another important factor for wild fish and those farmed in extensive systems is the productivity of the ecosystem which sustains their food supply. Since consideration of ecosystems and the ecophysiological requirements of fish is beyond the scope of the present text, the reader is referred to Rankin and Jensen (1993), Macan (1974) and Odum (1971).
PHYSICAL AND CHEMICAL ASPECTS OF WATER QUALITY
TEMPERATURE
Fish have upper and lower thermal tolerance limits and optimum temperatures for growth, egg incubation, food conversion and resistance to specific diseases. These optima vary with species and may be different for different parameters such as oxygen tension and water pH. Fresh waters are subject to temperature fluctuations of up to 40°C caused by latitude, season, altitude, time of day, depth and other factors. The range of temperature change of sea-waters is much less, due to water circulation in the seas and oceans and the large volumes of water involved.
Many fish diseases are temperature modulated, with pathogenicity closely related to a specific temperature range. In many host–pathogen systems there is a balance between the host’s defences and the pathogen’s invasiveness, but this is readily modified by temperature change, especially if it is rapid.
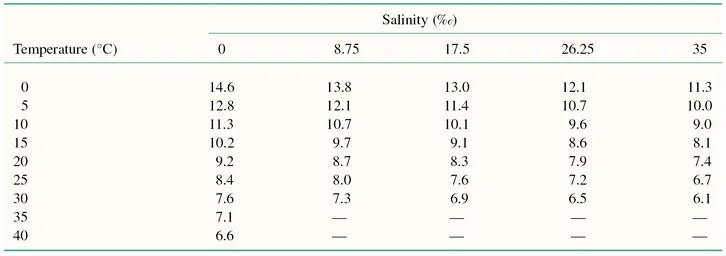
Water temperature also affects other properties of the aquatic environment important for fish health. Dissolved gases generally decrease in solubility with increasing temperature (Table 1.1), whereas the solubility of toxic compounds, which are only sparingly soluble in water, such as crude oil and pesticides, increases with temperature rise. The toxicity of some substances such as heavy metals also increases with temperature (Wedemeyer 1997).
Table 1.1 Solubility of oxygen in water exposed to water-saturated air* (mg/litre).
LIGHT
Light is a complex ecological factor whose components include colour spectrum (quality), intensity (quantity) and photoperiod (periodicity). The aquatic environment has peculiar and extremely variable characteristics, and ‘receptivity’ of fish to light changes profoundly from one species to another and, within the same species, from one developmental stage to another (Boeuf & Falcon 2001).
In natural waters and extensive farming systems, light levels can be changed only indirectly by methods such as increasing water depth and controlling unicellular algae, macrophytes and tree shade. Poor light penetration caused by absorbent or reflecting pollutants, such as clays, coal washings and paper wastes, diminishes algal productivity and may decrease the available levels of food for fish.
In intensive systems the light regime – intensity, photoperiod, shaded areas and light absorption by background – is more readily controlled. All of these parameters may contribute to aspects of the growth and maturation rate of fish. In such intensive culture systems, however, there is increasing evidence to suggest that ultraviolet light from excessive sunlight can result in sunburn of the dorsal surface, head or fins (Bullock 1987). With the development of thinning of the ozone layer as a result of excessive release of chlorinated compounds into the atmosphere, the problem of sunburn has become particularly serious in fish reared in the southern hemisphere. In addition, under certain conditions, even low levels of ultraviolet radiation can induce sunburn effects. This condition, known as photosensitisation, usually results from the incorporation in the diet of photoactive chemicals, often derived from drugs incorporated for therapeutic purposes or specific feed components such as porphyrins. In many cultured species, appropriate circadian periods of darkness and light are needed for proper timing and completion of events such as smoltification and sexual maturation, and good welfare and homeostasis often depend on appropriate photoperiod signals (Fjelldal, et al. 2005; Berril, et al. 2006).
PHYSICOCHEMICAL PARAMETERS
The Ionic Product of Water
Water behaves as a weak base and acid in that it is capable of losing or gaining a proton:
The equilibrium constant for the dissociation of water is given by
where CH+ and COH– are the respective ion concentrations. Experimental evidence has shown that in pure water at 25°C,
and since both ions are present in equal amounts,
pH and the pH Scale
In dilute aqueous solutions, the pH is defined as the negative logarithm of the hydrogen ion concentration. This is usually written as
Using our previous example, the pH of pure water at 25°C is
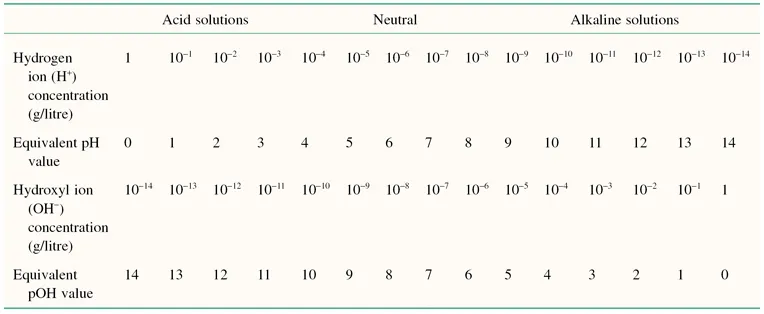
In dilute aqueous solutions the ion product of water is constant at 1 × 10−14. Additions of hydrogen and hydroxyl ions do not change this constant. The interrelations between hydrogen and hydroxyl ion concentration and their equivalent pH and pOH values are shown in Table 1.2. From this it is apparent that the pH scale varies from 0 to 14.
Table 1.2 Interrelationships between pH value and the hydrogen and hydroxyl ion concentrations in a dilute aqueous solution.
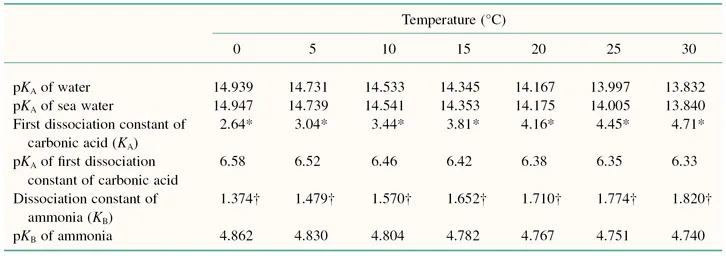
The pH scale is a negative logarithmic scale, meaning that for a decrease of 1 pH unit there is a 10-fold increase of hydrogen ion concentration. Neutrality on the pH scale is the point where equal amounts of hydrogen and hydroxyl ions exist. This value changes with the salt content and the temperature (Table 1.3). Where hydrogen ions are in excess of hydroxyl ions the solution is said to be acidic, and in the reverse situation it is called alkaline. To calculate the actual hydrogen ion concentration at, for example, a pH of 6.6,
Table 1.3 Effects of temperature on selected dissociation constants.
By taking the antilogarithm of both sides,
the number −6.6 can be re-expressed as 0.4–7.0. The antilog of 0.4 = 2.5 and the antilog of −7.0 = 10−7. Therefore,
and the reverse, to calculate the pH of a solution containing 3.98 × 10−8 g ions/litre H+:
Ionisation Constants of Weak Acids and Bases
Applying the law of mass action to the ionisation of a weak acid,
we have
where KA is the ionisation or dissociation constant of the weak acid. The stronger the acid, the greater the proportion of ions to undissociated molecules and the larger the value of KA. Because these values are very small, it is usual to express them as negative logarithms and to use the designation pKA, analogous to pH:
Weak bases such as ammonium hydroxide behave in a similar manner:
where KB is a measure of the extent to which a weak base dissociates hydroxyl ions. Converting the dissociation constant KB to its negative logarithm yields the constant pKB. The constants KB and pKB of weak bases relate to the hydroxyl ion concentrations and pOH values. Because we customarily express reactions even in alkaline solution in terms of pH rather than pOH, it is convenient to use the constant pKAB which relates the dissociation of a weak ...