
eBook - ePub
Pharmaceutical Biotechnology
Concepts and Applications
Gary Walsh
This is a test
Buch teilen
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Pharmaceutical Biotechnology
Concepts and Applications
Gary Walsh
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
Pharmaceutical Biotechnology offers students taking Pharmacy and related Medical and Pharmaceutical courses a comprehensive introduction to the fast-moving area of biopharmaceuticals. With a particular focus on the subject taken from a pharmaceutical perspective, initial chapters offer a broad introduction to protein science and recombinant DNA technology- key areas that underpin the whole subject. Subsequent chapters focus upon the development, production and analysis of these substances. Finally the book moves on to explore the science, biotechnology and medical applications of specific biotech products categories. These include not only protein-based substances but also nucleic acid and cell-based products.
- introduces essential principles underlining modern biotechnology- recombinant DNA technology and protein science
- an invaluable introduction to this fast-moving subject aimed specifically at pharmacy and medical students
- includes specific 'product category chapters' focusing on the pharmaceutical, medical and therapeutic properties of numerous biopharmaceutical products.
- entire chapter devoted to the principles of genetic engineering and how these drugs are developed.
- includes numerous relevant case studies to enhance student understanding
- no prior knowledge of protein structure is assumed
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Pharmaceutical Biotechnology als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Pharmaceutical Biotechnology von Gary Walsh im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Physical Sciences & Industrial & Technical Chemistry. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
1
Pharmaceuticals, biologics and biopharmaceuticals
1.1 Introduction to pharmaceutical products
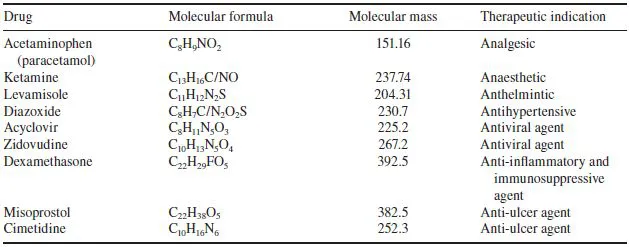
Pharmaceutical substances form the backbone of modern medicinal therapy. Most traditional pharmaceuticals are low molecular weight organic chemicals (Table 1.1). Although some (e.g. aspirin) were originally isolated from biological sources, most are now manufactured by direct chemical synthesis. Two types of manufacturing company thus comprise the ‘traditional’ pharmaceutical sector: the chemical synthesis plants, which manufacture the raw chemical ingredients in bulk quantities, and the finished product pharmaceutical facilities, which purchase these raw bulk ingredients, formulate them into final pharmaceutical products, and supply these products to the end user.
In addition to chemical-based drugs, a range of pharmaceutical substances (e.g. hormones and blood products) are produced by/extracted from biological sources. Such products, some major examples of which are listed in Table 1.2, may thus be described as products of biotechnology. In some instances, categorizing pharmaceuticals as products of biotechnology or chemical synthesis becomes somewhat artificial. For example, certain semi-synthetic antibiotics are produced by chemical modification of natural antibiotics produced by fermentation technology.
1.2 Biopharmaceuticals and pharmaceutical biotechnology
Terms such as ‘biologic’, ‘biopharmaceutical’ and ‘products of pharmaceutical biotechnology’ or ‘biotechnology medicines’ have now become an accepted part of the pharmaceutical literature. However, these terms are sometimes used interchangeably and can mean different things to different people.
Although it might be assumed that ‘biologic’ refers to any pharmaceutical product produced by biotechnological endeavour, its definition is more limited. In pharmaceutical circles, ‘biologic’ generally refers to medicinal products derived from blood, as well as vaccines, toxins and allergen products. ‘Biotechnology’ has a much broader and long-established meaning. Essentially, it refers to the use of biological systems (e.g. cells or tissues) or biological molecules (e.g. enzymes or antibodies) for/in the manufacture of commercial products.
Table 1.1 Some traditional pharmaceutical substances that are generally produced by direct chemical synthesis
The term ‘biopharmaceutical’ was first used in the 1980s and came to describe a class of therapeutic proteins produced by modern biotechnological techniques, specifically via genetic engineering (Chapter 3) or, in the case of monoclonal antibodies, by hybridoma technology (Chapter 13). Although the majority of biopharmaceuticals or biotechnology products now approved or in development are proteins produced via genetic engineering, these terms now also encompass nucleic-acid-based, i.e. deoxyribonucleic acid (DNA)- or ribonucleic acid (RNA)-based products, and whole-cell-based products.
1.3 History of the pharmaceutical industry
The pharmaceutical industry, as we now know it, is barely 60 years old. From very modest beginnings, it has grown rapidly, reaching an estimated value of US$100 billion by the mid 1980s. Its current value is likely double or more this figure. There are well in excess of 10 000 pharmaceutical companies in existence, although only about 100 of these can claim to be of true international significance. These companies manufacture in excess of 5000 individual pharmaceutical substances used routinely in medicine.
Table 1.2 Some pharmaceuticals that were traditionally obtained by direct extraction from biological source material. Many of the protein-based pharmaceuticals mentioned are now also produced by genetic engineering
| Substance | Medical application |
| Blood products (e.g. coagulation factors) | Treatment of blood disorders such as haemophilia A or B |
| Vaccines | Vaccination against various diseases |
| Antibodies | Passive immunization against various diseases |
| Insulin | Treatment of diabetes mellitus |
| Enzymes | Thrombolytic agents, digestive aids, debriding agents (i.e. cleansing of wounds) |
| Antibiotics | Treatment against various infections agents |
| Plant extracts (e.g. alkaloids) | Various, including pain relief |
The first stages of development of the modern pharmaceutical industry can be traced back to the turn of the twentieth century. At that time (apart from folk cures), the medical community had at their disposal only four drugs that were effective in treating specific diseases:
- Digitalis (extracted from foxglove) was known to stimulate heart muscle and, hence, was used to treat various heart conditions.
- Quinine, obtained from the barks/roots of a plant (Cinchona genus), was used to treat malaria.
- Pecacuanha (active ingredient is a mixture of alkaloids), used for treating dysentery, was obtained from the bark/roots of the plant genus Cephaelis.
- Mercury, for the treatment of syphilis.
This lack of appropriate, safe and effective medicines contributed in no small way to the low life expectancy characteristic of those times.
Developments in biology (particularly the growing realization of the microbiological basis of many diseases), as well as a developing appreciation of the principles of organic chemistry, helped underpin future innovation in the fledgling pharmaceutical industry. The successful synthesis of various artificial dyes, which proved to be therapeutically useful, led to the formation of pharmaceutical/chemical companies such as Bayer and Hoechst in the late 1800s. Scientists at Bayer, for example, succeeded in synthesizing aspirin in 1895.
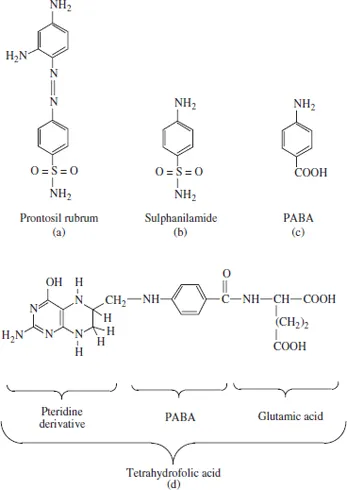
Despite these early advances, it was not until the 1930s that the pharmaceutical industry began to develop in earnest. The initial landmark discovery of this era was probably the discovery, and chemical synthesis, of the sulfa drugs. These are a group of related molecules derived from the red dye prontosil rubrum. These drugs proved effective in the treatment of a wide variety of bacterial infections (Figure 1.1). Although it was first used therapeutically in the early 1920s, large-scale industrial production of insulin also commenced in the 1930s.
The medical success of these drugs gave new emphasis to the pharmaceutical industry, which was boosted further by the commencement of industrial-scale penicillin manufacture in the early 1940s. Around this time, many of the current leading pharmaceutical companies (or their forerunners) were founded. Examples include Ciba Geigy, Eli Lilly, Wellcome, Glaxo and Roche. Over the next two to three decades, these companies developed drugs such as tetracyclines, corticosteroids, oral contraceptives, antidepressants and many more. Most of these pharmaceutical substances are manufactured by direct chemical synthesis.
1.4 The age of biopharmaceuticals
Biomedical research continues to broaden our understanding of the molecular mechanisms underlining both health and disease. Research undertaken since the 1950s has pinpointed a host of proteins produced naturally in the body that have obvious therapeutic applications. Examples include the interferons and interleukins (which regulate the immune response), growth factors, such as erythropoietin (EPO; which stimulates red blood cell production), and neurotrophic factors (which regulate the development and maintenance of neural tissue).
Figure 1.1 Sulfa drugs and their mode of action. The first sulfa drug to be used medically was the red dye prontosil rubrum (a). In the early 1930s, experiments illustrated that the administration of this dye to mice infected with haemolytic streptococci prevented the death of the mice. This drug, although effective in vivo, was devoid of in vitro antibacterial activity. It was first used clinically in 1935 under the name Streptozon. It was subsequently shown that prontosil rubrum was enzymatically reduced by the liver, forming sulfanilamide, the actual active antimicrobial agent (b). Sulfanilamide induces its effect by acting as an anti-metabolite with respect to para-aminobenzoic acid (PABA) (c). PABA is an essential component of tetrahydrofolic acid (THF) (d). THF serves as an essential cofactor for several cellular enzymes. Sulfanilamide (at sufficiently high concentrations) inhibits manufacture of THF by competing with PABA. This effectively inhibits essential THF-dependent enzyme reactions within the cell. Unlike humans, who can derive folates from their diets, most bacteria must synthesize it de novo, as they cannot absorb it intact from their surroundings
Although the pharmaceutical potential of these regulatory molecules was generally appreciated, their widespread medical application was in most cases rendered impractical due to the tiny quantities in which they were naturally produced. The advent of recombinant DNA technology (genetic engineering) and monoclonal antibody technology (hybridoma technology) overcame many such difficulties, and marked the beginning of a new era of the pharmaceutical sciences.
Recombinant DNA technology has had a fourfold positive impact upon the production of pharmaceutically important proteins:
- It overcomes the problem of source availability. Many proteins of therapeutic potential are produced naturally in the body in minute quantities. Examples include interferons (Chapter 8), interleukins (Chapter 9) and colony-stimulating factors (CSFs; Chapter 10). This rendered impractical their direct extraction from native source material in quantities sufficient to meet likely clinical demand. Recombinant production (Chapters 3 and 5) allows the manufacture of any protein in whatever quantity it is required.
- It overcomes problems of product safety. Direct extraction of product from some native biological sources has, in the past, led to the unwitting transmission of disease. Examples include the transmission of blood-borne pathogens such as hepatitis B and C and human immunodeficiency virus (HIV) via infected blood products and the transmission of Creutzfeldt–Jakob disease to persons receiving human growth hormone (GH) preparations derived from human pituitaries.
- It provides an alternative to direct extraction from inappropriate/dangerous source material. A number of therapeutic proteins have traditionally been extracted from human urine. Follicle-stimulating hormone (FSH), the fertility hormone, for example, is obtained from the urine of post-menopausal women, and a related hormone, human chorionic gonadotrophin (hCG), is extracted from the urine of pregnant women (Chapter 11). Urine is not considered a particularly desirable source of pharmaceutical products. Although several products obtained from this source remain on the market, recombinant forms have now also been approved. Other potential biopharmaceuticals are produced naturally in downright dangerous sources. Ancrod, for example, is a protein displaying anti-coagulant activity (Chapter 12) and, hence, is of potential clinical use. It is, however, produced naturally by the Malaysian pit viper. Although retrieval by milking snake venom is possible, and indeed may be quite an exciting procedure, recombinant production in less dangerous organisms, such as Escherichia coli or Saccharomycese cerevisiae, would be considered preferable by most.
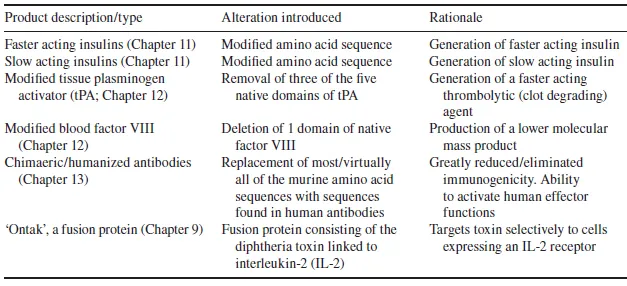
- It facilitates the generation of engineered therapeutic proteins displaying some clinical advantage over the native protein product. Techniques such as site-directed mutagenesis facilitate the logical introduction of predefined changes in a protein’s amino acid sequence. Such changes can be as minimal as the insertion, deletion or alteration of a single amino acid residue, or can be more substantial (e.g. the alteration/deletion of an entire domain, or the generation of a novel hybrid protein). Such changes can be made for a number of reasons, and several engineered products have now gained marketing approval. An overview summary of some engineered product types now on the market is provided in Table 1.3. These and other examples will be discussed in subsequent chapters.
Despite the undoubted advantages of recombinant production, it remains the case that many protein-based products extracted directly from native source material remain on the market. In certain circumstances, direct extraction of native source material can prove equally/more attractive than recombinant production. This may be for an economic reason if, for example, the protein is produced in very large quantities by the native source and is easy to extract/purify, e.g. human serum albumin (HSA; Chapter 12). Also, some blood factor preparations purified from donor blood actually contain several different blood factors and, hence, can be used to treat several haemophilia patient types. Recombinant blood factor preparations, on the other hand, contain but a single blood factor and, hence, can be used to treat only one haemophilia type (Chapter 12).
The advent of genetic engineering and monoclonal antibody technology underpinned the establishment of literally hundreds of start-up biopharmaceutical (biotechnology) companies in the late 1970s and early 1980s. The bulk of these companies were founded in the USA, with smaller numbers of start-ups emanating from Europe and other world regions.
Table 1.3 Selected engineered biopharmaceutical types/products that have now gained marketing approval. These and additional such products will be discussed in detail in subsequent chapters
Many of these fledgling companies were founded by academics/technical experts who sought to take commercial advantage of developments in the biotechnological arena. These companies were largely financed by speculative monies attracted by the hype associated with the establishment of the modern biotech era. Although most of these early companies displayed significant technical expertise, the vast majority lacked experience in the practicalities of the drug development process (Chapter 4). Most of the well-established large pharmaceutical companies, on the other hand, were slow to invest heavily in biotech research and development. However, as the actual and potential therapeutic significance of biopharmaceuticals became evident, many of these companies did diversify into this area. Most either purchased small, established biopharmaceutical concerns or formed strategic alliances with them. An example was the long-term alliance formed by Genentech (see later) and the well-established pharmaceutical company Eli Lilly. Genentech developed recombinant human insulin, which was then marketed by Eli Lilly under the trade name Humulin. The merger of biotech capability with pharmaceutical experience helped accelerate development of the biopharmaceutical sector.
Table 1.4 Pharmaceutical companies who manufacture and/or market biopharmaceutical products approved for general medical use in the USA and EU
| Sanofi-Aventis | Hoechst AG |
| Bayer | Wyeth |
| Novo Nordisk | Genzyme |
| Isis Pharmaceuticals | Abbott |
| Genentech | Roche |
| Centocor | Novartis |
| Boehringer Manheim | Serono |
| Galenus Manheim | Organon |
| Eli Lilly | Amgen |
| Ortho Biotech | GlaxoSmithKline |
| Schering Plough | Cytogen |
| Hoffman-la-Roche | Immunomedics |
| Chiron | Biogen |
Many of the earlier biopharmaceutical companies no longer exist. The overall level of speculative finance available was not sufficient to sustain them all long term (it can take 6–10 years and US$800 million to develop a single drug; Chapter 4). Furthermore, the promise and hype of biotechnology sometimes exceeded its ability actually to deliver a final product. Some biopharmaceutical substances showed little efficacy in treating their target condition, and/or exhibited unacceptable side effects. Mergers and acquisitions also led to the disappearance of several biopharmaceutical concerns. Table 1.4 lists many of the major pharmaceutical concerns which now manufacture/market biopharmaceuticals approved for general medical use. Box 1.1 prov...