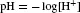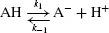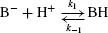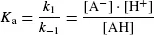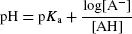![]()
Chapter 1
Introduction
Objectives
After completing this chapter you should be familiar with:
- The special chemical conditions often required by biomolecules.
- Importance of the use of buffers in the study of physical phenomena in biochemistry.
- Quantification of physical phenomena.
- Objectives of this volume.
This volume describes a range of physical techniques which are now widely used in the study both of biomolecules and of processes in which they are involved. There will be a strong emphasis throughout on biomacromolecules such as proteins and nucleic acids as well as on macromolecular complexes of which they are components (e.g. biological membranes, ribosomes, chromosomes). This is because such chemical entities are particularly crucial to the correct functioning of living cells and present specific analytical problems compared to simpler biomolecules such as monosaccharides or dipeptides. Biophysical techniques, give detailed information offering insights into the structure, dynamics and interactions of biomacromolecules.
Life scientists in general and biochemists in particular have devoted much effort during the last century to elucidation of the relationship between structure and function and to understanding how biological processes happen and are controlled. Major progress has been made using chemical and biological techniques which, for example, have contributed to the development of the science of molecular biology. However, in the last decade physical techniques which complement these other approaches have seen major development and these now promise even greater insight into the molecules and processes which allow the living cell to survive. For example, a major focus of life science research currently is the proteome as distinct from the genome. This has emphasized the need to be able to study the highly-individual structures of biomacromolecules such as proteins to understand more fully their particular contribution to the biology of the cell. For the foreseeable future, these techniques are likely to impact to a greater or lesser extent on the activities of most life scientists. This text attempts to survey the main physical techniques and to describe how they can contribute to our knowledge of biological systems and processes. We will set the scene for this by first looking at the particular analytical problems posed by biomolecules.
1.1 SPECIAL CHEMICAL REQUIREMENTS OF BIOMOLECULES
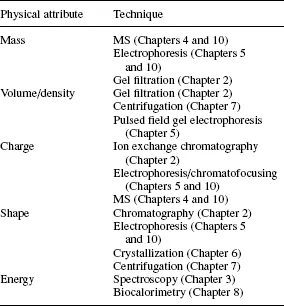
The tens of thousands of biomolecules encountered in living cells may be classified into two general groups. Biomacromolecules (e.g. proteins; nucleic acids) are characterized by high molecular mass (denoted throughout this text as relative molecular mass, Mr) and are generally unstable under extreme chemical conditions where they may lose structure or break down into their chemical building blocks. Low molecular weight molecules are smaller and more chemically robust (e.g. amino acids; nucleotides; fatty acids). Within each group there is displayed a wide range of water-solubility, chemical composition and reactivity which is determined by complex interactions between physicochemical attributes of the biomolecule and solvent. These attributes are the main focus for the techniques described in this volume and reflect the highly individual function which each molecule performs in the cell (Tables 1.1 and 1.2).
Notwithstanding the great range of form and structure, we can nonetheless recognize certain attributes as common to all biomolecules. The first and most obvious is that all of these molecules are produced in living cells under mild chemical conditions of temperature, pressure and pH. Biomacromolecules are built up from simpler building block molecules by covalent bonds formed usually with the elimination of water. Moreover, biomolecules are continuously synthesized and degraded in cells in a highly regulated manner. It follows from this that many biomolecules are especially sensitive to extremes of temperature and pH which may present a problem in their handling prior to and during any biophysical analysis. Since biomolecules result from a long process of biological evolution during which they have been selected to perform highly specific functions, a very close relationship has arisen between chemical structure and function. This means that, even at pH and temperature values under which the molecule may not be destroyed, it may function suboptimally or not at all.
Table 1.1. Some important physical attributes of biomolecules amenable to study by biophysical techniques
Table 1.2. Some important chemical attributes of biomolecules which may be used in study by biophysical techniques
These facts impose limitations on the chemical conditions to which biomolecules may be exposed during extraction, purification or analysis. In most of the techniques described in this volume, sample analytes are exposed to a specific set of chemical conditions by being dissolved in a solution of defined composition. Whilst other components in the solution may also be important in individual cases as will be discussed below (Section 1.3.2), three main variables govern the makeup of this solution which are discussed in more detail in the following section.
1.2 FACTORS AFFECTING ANALYTE STRUCTURE AND STABILITY
In practice, most of the biophysical procedures described in this volume use conditions which have been optimized over many years for thousands of different samples. These robust conditions will normally maintain the sample in a defined structural form facilitating its separation and/or analysis. However, some procedures (e.g. chromatography, capillary electrophoresis, crystallization) may require case-by-case optimization of conditions. Before embarking on a detailed analysis of a biomolecule using biophysical techniques it is often useful to know something about the stability of the sample to chemical variables, especially pH, temperature and solvent polarity. This knowledge can help us to design a suitable solvent or set of chemical conditions which will maximize the stability of the analyte for the duration of the experiment and may also help us to explain unexpected results. For example, we sometimes find loss of enzyme activity during column chromatography which may be partly explained by the chemical conditions experienced by the protein during the experiment. Moreover, many of the techniques described in this volume are actually designed to be suboptimal and to take advantage of disruption of the normal functional structure of the biomolecule to facilitate separation or analysis (e.g. electrophoresis, HPLC, MS).
A good indication of the most stabilizing conditions may often be obtained from knowledge of the biological origin of the biomolecule. It is also wise to assess the structural and functional stability of the analyte over the range of experimental conditions encountered in the experiment during its likely time-span.
We can distinguish two main types of effects as a result of variation in the chemical conditions to which biomolecules are exposed. Structural effects reflect often irreversible structural change in the molecule (e.g. protein/nucleic acid denaturation; hydrolysis of covalent bonds between building blocks of which biopolymers are composed). Functional effects are frequently more subtle and may be reversible (e.g. deprotonation of chemical groups in the biomolecule resulting in ionization; partial unfolding of proteins). A detailed treatment of these effects on the main classes of biomolecules is outside the scope of the present volume but a working knowledge of the likely effects of these conditions can be very useful in deciding conditions for separation or analytical manipulation.
1.2.1 pH Effects
pH is defined as the negative log of the proton concentration:
Because both the H+ and OH− concentrations of pure water are 10–7 M, this scale runs from a maximum of 14 (strongly alkaline) to a minimum of 0 (strongly acidic). As it is a log scale, one unit reflects a 10-fold change in proton concentration. Most biomacromolecules are labile to alkaline or acid-catalyzed hydrolysis at extremes of the pH scale but are generally stable in the range 3–10. It is usual to analyse such biopolymers at pH values where they are structurally stable and this may differ slightly for individual biopolymers. For example, proteins normally expressed in lysosomes (pH 4) are quite acid-stable while those from cytosol (pH 7) may be unstable near pH 3. Aqueous solutions in which sample molecules are dissolved usually comprise a buffer to prevent changes in pH during the experiment. These are described in more detail in Section 1.3 below.
Many biomolecules are amphoteric in aqueous solution that is they can accept or donate protons. Some chemical groups such as inorganic phosphate or acidic amino acid side-chains (e.g. aspartate) can act as Brønsted acids and donate protons:
Other groups such as the imidazole ring of histidine or amino groups can act as Brønsted bases and accept protons
The position of equilibrium in these protonation/ deprotonation events may be described by an equilibrium constant, Ka:
pKa (–logKa) is the pH value at which 50% of the acid is protonated and 50% is deprotonated. The Henderson–Hasselbach equation describes variation of concentrations of A− and AH as a function of pH:
Functional groups present as structural components of biomolecules (e.g. amino acid side-chains of proteins; phosphate groups of nucleotides) will have distinct Ka values which may differ slightly from the value found in other chemical circumstances (e.g. the Ka values of amino acid side-chains in polypeptides differ from those in the free amino acid). Some biomolecules can contain both acidic and basic groups within their structure (e.g. proteins) while particular chemical structures found in biomolecules may be polyprotic, that is capable of multiple ionizations (e.g. phosphate). Such biomolecules may undergo a complex pattern of ionization resulting in varying net charge on the molecule. pH titration curves for biomolecules allow us to identify pKa values (Figure 1.1).
Since protonation-deprotonation effects are responsible for the charges on biomacromolecules which maintain their solubility in water, their solubility is often lowest at their isoelectric point, pI, the pH value at which the molecule has no net charge. These can also be determined by titration using methods described in Chapter 5 (Section 5.5.3; Figure 5.2...