![]()
The interaction of polymers with different materials such as metals, ceramics, other polymers, coatings, or inorganics is crucial for the adhesion at interfaces in polymer composite structural elements. The absence or weakness of interactions as well as any lack of durability are responsible for the collapse of load-bearing composite components. In 2005 the ice rink in Bad Reichenhall (Germany) collapsed, burying several people, because of adhesion failure (fatigue of the interface bonds).
Many polymers, in particular polyolefins, such as polyethylene and polypropylene are chemically inert and cannot strongly interact with other materials. The reason for this is the absence of polar and reactive functional groups in their structure. Thus, interactions with other materials are poor and so too is adhesion. Weak physical interactions only occur. J. D. van der Waals found their existence in 1879 [1]. These forces are electrostatic, induced and permanent dipoles, dispersion interactions, and hydrogen bonds. They are very weak and operate over a short range [2]. Polyolefins show only dispersion interactions among their own molecules and, thus, they are often difficult to wet or bond because of the absence of polar groups, which are able to promote interactions to the other material. Dipole or induced-dipole interactions or even chemical bonds between polymer and coating at the interface require the existence of functional groups.
Polar groups are often introduced by flaming [3] or plasma exposure [4]. Such oxidations form various oxidized polar species at the polyolefin surface, which can undergo the desired interactions to other materials. The introduction of chemical bonds at the interface is more efficient because of the much higher binding energies [5]. To install such covalent bonds between polymers and coatings, most often the production of monotype functional groups at the polyolefin surface is a necessary precondition. Such monosort functionalization is extraordinarily difficult. New processes have been developed for its realization, that is, exposure of the polyolefin surface to brominating plasma [6]. The C–Br groups could be converted into amino, carboxyl, or hydroxyl groups or consumed by amines, alcohols, and glycols [7]. The additional introduction of flexible, water-repellent, and metal-binding spacer molecules by grafting onto C–Br groups produced highly adhered and durable polyolefin composites [8].
In highly stressed polymer components for structural assemblies all forces are applied to the interface and distributed to the interfacial bonds. Either a large number of weak physical interactions or a smaller number of strong chemical bonds is needed to withstand the disruption under mechanical load along the interface. However, in general, chemically and structurally, completely different materials need to be joined together. Polymers, in particular polyolefins, show very low surface energy and metals or inorganics a much higher one. The difference amounts to two orders of magnitude (original value for polymers 30–40 mN m−1 and for metals 1000–3000 mN m−1), which is nearly the same difference in surface energy as before polymer treatment (40–50 and 1000–3000 mN m−1) [9]. At the molecular level, interactions are absent due to the chemical inertness of polyolefins.
Post-polymerization introduction of functional groups onto polyolefin surfaces has a principal problem. The (radical) substitution of H by any functional group is accompanied by C–C bond scissions of the polymer backbone because of equivalent (or lower) binding energies [10]. Thus, degradation occurs simultaneously, although C–C bonds were partially shielded from attack. Nevertheless, such a disruption of the polymer surface produces anchoring points for physical and chemical interactions but also a weak boundary layer, which is mechanically, chemically, and thermally unstable (low molecular weight oxidized material, LMWOM) [11]. Moreover, polymers, metals, or inorganics have thermal expansion coefficients that differ by two orders of magnitude. Therefore, the thus produced mechanical stress is focused onto the monolayer of interactions along the interface. As mentioned before, spacer introduction can balance this mechanical stress along the interface.
The surface modification of polyolefins must be also considered within the framework of 100 Mio tons production of polyethylene and polypropylene per year worldwide. Several technical applications demand a solution to the adhesion problem. Mechanical interlocking, chemical roughening by etching, ion and electron beam modification, UV irradiation, UV-induced graft copolymerization, laser beam or excimer lamp irradiation, 60Co irradiation, flaming, corona treatment, use of adhesion promoters, glues, adhesives, etc. were successfully tested to modify polyolefin surfaces for adhesion [2]. However, all these pretreatments produce a broad variety of different functional groups.
As mentioned before, the formation of monotype functional groups followed by spacer grafting can solve the problem of moderate adhesive bond strength and durability. However, the great energy and enthalpy excess present in a plasma is most often responsible for non-selective reactions and the formation of a broad variety of products [12].
The dream of all plasma chemists is to achieve monosort functionalized polyolefin surfaces. The excess energy present in the plasma state [13] and the equivalency of C–C and C–H dissociation energies make it difficult to realize this dream [10]. However, a few chemical reactions produce end-products that are also stable towards plasma. Examples of such stable end-products are (i) in the case of bromination the electronic state of the neighboring noble gas (krypton) and (ii) silica-like SiOx layers formed in the oxidation of Si compounds in an oxygen plasma [14].
This book presents several variants of such surface techniques with monotype functional groups, such as chemical post-plasma reduction, pulse-pressure plasma polymerization, underwater plasma and glow discharge electrolysis, and deposition of functionalized prepolymers and oligomers by aerosol plasma and electrospray [15].
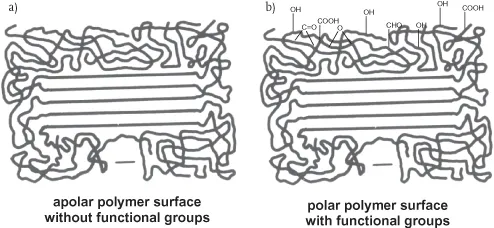
Polyolefins have a semi-crystalline structure, which can be represented by the model of “Fransenmicelle” as shown in Figure 1.1.
Amorphous regions are characterized by random localization of macromolecular chains, whereas crystalline regions show the parallel and close orientation of the all-trans configuration of the chain with folded loops, thus forming the lamellae as present in polyethylene [16].
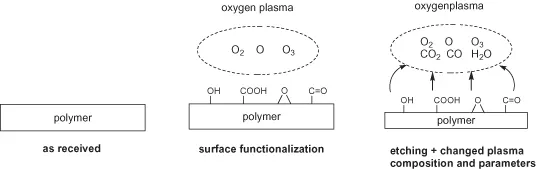
The concept of polymer functionalization by plasma exposure is to attach atoms or fragments of the dissociated plasma gas as functional group by H substitution at the polymer chain. Since there are there many different fragments and atoms present in the plasma a broad variety of related functional groups is produced. The formation of at least 12 oxygen-containing groups at the surface of poly(ethylene terephthalate) has been shown after oxygen plasma exposure [17].
There is also an interrelation between plasma, polymer, surface charging, surface cleaning, surface functionalization, etching, and emission of degradation products as well as changing of plasma by the appearance of oxygen-containing groups in the gas phase and so on (Figure 1.2).
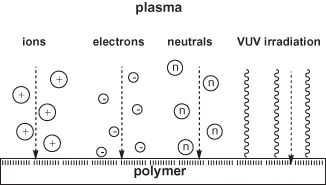
The substrate, here the polymer, gives a specific response to plasma exposure. Polymers react very sensitively to any exposure to plasmas. This is due to their complex and supermolecular structure. Polymers have some common features with living matter and therefore they are very sensitive, in almost the same manner, towards particle or radiation exposure. Thus, special knowledge of polymer chemistry, physics, and technology is necessary to understand the specific and complex behavior of polymer surfaces on plasma exposure. Starting from plasma physics and taking simple atomic (noble) or molecule gas plasmas, which are well-defined and well-characterized but, nevertheless, are associated with high power consumption and high average electron energy the contradictoriness of flow from plasma to polymer, thus the confrontation is perfect. A shower of high-energy particles and photons bombards the polymer surface. A result of this bombardment is the formation of degraded or crosslinked products with the complete loss of original structure (Figure 1.3) [18].
As a matter of course, as a precondition, the plasma gas temperatures should be near room temperature or, in the case of energy-rich hot plasmas, a very short residence time in the plasma zone is mandatory. Low gas temperature is characteristic for low-pressure glow discharges, also known as non-isothermal plasmas or colloquially as “cold” plasmas [19]. Figure 1.4 shows schematically the prototype of such a plasma, namely, the low-pressure DC (direct current) glow discharge. The volume between the two electrodes is filled with the uniform plasm...