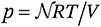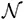![]()
CHAPTER 1
Introduction
We briefly discuss the occurrence and importance of dispersion (van der Waals) interactions. We then give a historical review of the important developments in the understanding and modelling of these interactions, from the 19th century to the present day.
1.1 General Introduction
The four basic forces in nature (strong nuclear, weak nuclear, electromagnetic and gravitational) have very different strengths. Most of the forces that we experience every day, except gravity, are ultimately electromagnetic in origin. Intrinsically, gravity is the weakest force by far: for two protons the ratio of gravitational attraction to electrostatic repulsion is of order 10−36 and for two electrons it is of order 10−43.
All living things are made of protons, neutrons and electrons to a first approximation and so are subject to electromagnetic forces yet, despite its relative weakness, gravity is very important to us, as anyone who has fallen over can attest. Indeed it is said that an elephant experiences very serious injuries from a fall of only 0.5 m.
The reason for this apparent contradiction is that electrostatic forces can be repulsive or attractive depending on the sign of the electric charges involved, and since living things are close to being electrically neutral overall, electrostatic forces from external objects very nearly cancel out. Static gravity, on the other hand, is always attractive and so cannot cancel out. The reason gravity is important to us is the enormous amount of matter (the Earth) that lies beneath our feet, together with the non-cancelling nature of the force.
Even the large size of the Earth does not always ensure dominance of the gravitational force for objects near its surface. Small creatures can fall without injury because of air friction, but have different problems including becoming trapped in any body of water by surface tension (an ultimately electromagnetic force).
At the nanoscale, it turns out that another aspect of electromagnetism, namely the dispersion (van der Waals, vdW) force, becomes very important. Although the dispersion force is weak compared with covalent and ionic forces, it is primarily attractive and so, like gravity at the macroscale, it can produce strong effects where (relatively) large amounts of nanoscale matter are present.
The analogy between dispersion and gravitational forces only goes so far, however, as vdW forces can occasionally be repulsive. Furthermore, the floorboards do not shield us from our gravitational attraction to the Earth beneath our feet. A contrasting situation can occur with dispersion forces, where a conducting graphene (carbon) layer one atom thick, inserted between two other nano-objects, has been shown 1-3 to “screen out” the vdW force between those objects. In keeping with the electromagnetic origin of vdW forces, this screening effect has been shown 2 to be analogous to the “Faraday cage” effect whereby electric fields are screened out by a metal cage.
1.2 What are Dispersion Forces?
Dispersion forces are weak forces that occur between any pieces of matter that contain electrons. They are usually but not always attractive. They are long-ranged in the sense that their decay with separation is via inverse powers of distance, rather than exponential decay as for covalent forces. The ultimate origin of these forces is electromagnetic, but their mechanism is quite subtle, as explained in the following paragraphs.
It is well known that two static electric charges attract or repel each other with a Coulomb force that decays with separation R as 1/R 2. For two particles that carry no net charge, but that carry a time-constant electric dipole moment, the electromagnetic force falls off as 1/R 4, has a strong dependence on the angular orientation of the dipoles, and can be attractive or repulsive (see Section 2.1). The interaction between higher static electric multipoles falls off with higher powers of R −1.
For a pair of spherical atoms, such as argon, that have no permanent electric moments,† it is found that there is nevertheless an attractive inter-atom dispersion force. This clearly cannot arise from any permanent electric moments. The simplest way to understand this is to postulate that quantum mechanical zero-point motions of the electrons give rise to short-lived dipole moments on each atom, moments that average to zero over time. It is the electromagnetic interaction between these transient dipoles that causes the dispersion interaction. This simple picture is expanded in Section 2.4.
Under some circumstances it is the thermal fluctuations of the electronic moments, rather than the zero point quantal fluctuations, that are predicted to dominate the dispersion force.
There are other ways to understand dispersion forces, for example by directly considering the zero-point energy of the electromagnetic field in the presence of the pieces of matter concerned, or from second order quantum mechanical perturbation theory of the combined electronic motions. These viewpoints are also discussed at an intuitive level in Sections 2.6 and 2.7 (ii). The rest of the book then develops various methods in full mathematical detail, with guidance for numerical implementation.
1.3 When are Dispersion Forces Important?
- Intermolecular interactions and scattering in gas phase chemistry.
- Intramolecular energetics and stereochemistry of larger molecules.
- Relative stability of hydrocarbon isomers.
- Cohesion of rare-gas and molecular crystals, the latter important in pharmaceuticals.
- Cohesion of layered solids such as MoS2, graphite, etc.
- Cohesion and self-assembly of nanostructures in general.
- Stiction in nano-machines.
- Liquid crystals.
- Stability and tertiary structure of biopolymers such as proteins and DNA.
- Mesoscale biological phenomena such as the sticking of gecko feet.
- Colloid properties.
- Aerosol properties, toxicity.
- Catalysis.
- Relative stability of proposed chemical reaction intermediates.
- Wetting analysis.
- Thin films: adhesion of paints and coatings.
- Surface science in general.
- Accurate quantification of vdW forces is required for analysis of experiments designed to seek the postulated fifth fundamental force of nature.
1.4 Historical Summary of Conceptual Developments
Good summaries of the history of intermolecular force studies have been given by Rowlinson 4 and Stone, 5 for example. Here we focus only on dispersion forces.
1.4.1 Late 19th Century: Discovery of vdW Forces
Our discussion begins in the
19th century, but the interested reader can find some material about precursor ideas in the book by Israelachvili.
6 By the middle of the 19th century the ideal gas law
was well established to describe the pressure
p of a gas in terms of the number
of moles, the absolute (Kelvin) temperature
T and the universal gas constant
R. This law was synthesised by Clapeyron from separate empirical laws for the dependence on volume, temperature and molar content. Eqn (1.1) was explained by Koenig and Clausius from kinetic theory. In this approach the pressure on the containing wall of a gas arises when the gas particles bounce off the wall and transfer momentum to it. If the average kinetic energy of the gas particles is assumed to be proportional to the absolute temperature
T, then (eqn (1.1)) follows after averaging over the particle–wall collisions. In this theory no interaction between the particles is either assumed or allowed.
Eqn (1.1) was known to become accurate at high temperatures and low densities, but modifications were clearly required to account for observed deviations such as condensation to a liquid at low temperatures and high densities.
Johannes Diderik van der Waals studied this problem and in 1873 he proposed a modification of eqn (1.1), the
van der Waals equation of state, in the form
Here b was in the first instance considered to be the volume occupied by the finite-sized particles constituting the gas, so that a reduced volume V − b is available for their motion. Short-ranged repulsive forces are thereby tacitly assumed to prevent penetration of one particle into the volume of another particle. The term a/V 3 reduces the pressure, and was motivated by assuming the existence of a long-ranged attractive force between the gas particles, a force that reduces the pressure exerted on the walls of the container by those particles that are near the walls.
Eqn (1.2) proved successful in describing gases over a wide range of conditions, even giving qualitatively reasonable results near the critical point. Even quantitative agreement was possible in many regimes if the parameters a and b were allowed to depend on volume and temperature. van der Waals received the physics Nobel prize in 1910 for his work on non-ideal gases.
It is interesting to recall that, even in the late 19th century when eqn (1.2) was proposed, many scientists did not accept the reality of the particles (atoms and molecules) that are needed to explain the kinetic pressure of dilute gases from a microscopic point of view. While the reduction of pressure on the walls via intra-gas attraction could perhaps be obtained in a continuum model without postulating discrete particles in the gas, van der Waals was a staunch supporter of the existence of atoms and molecules (see for example his 1910 Nobel prize address 7 ). Because of this, his notion of attractive forces within a gas had implications far beyond the macroscopic properties of gases. It implied that all species (atoms or molecules) experience forces attracting them to each other. This attraction does not depend on the species having permanent charges, or permanent dipole or multipole electric moments. This is evidenced by the success of the van der Waals equation of state for (e.g.) the noble gases He, Ar, Kr etc., whose atoms have no such moments.
From a modern point of view, the numbers obtained for the attraction parameter a in eqn (1.2) show that the attractive part of van der Waals' universal force is much weaker than covalent and i...