
eBook - ePub
Glucuronidation of Drugs and Other Compounds
Geoffrey Dutton
This is a test
Buch teilen
- 268 Seiten
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Glucuronidation of Drugs and Other Compounds
Geoffrey Dutton
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
Published in 1980: In a previous publication on glucuronic acid both free and conjugated, the author expressed the hope that glucuronic acid studies over the following few years might expand vigorously. The have expanded, and none more vigorously that the study of biosynthesis of simple glucuronides.
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Glucuronidation of Drugs and Other Compounds als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Glucuronidation of Drugs and Other Compounds von Geoffrey Dutton im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Medizin & Pharmakologie. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
Part I
Glucuronidation, Glucuronides, and Studies on UDPGlucuronyltransferase In Vitro
Chapter 1
Introduction — The Biological Function of Glucuronidation
I. What Glucuronidation Is
Glucuronidation is the most widespread form of “conjugation” in mammalian metabolism. “Conjugation” is a synthetic reaction involving the coupling in vitro of two molecules, usually with elimination of water. There are ten major conjugation reactions in mammals,2 and many others exist, in both animals and plants.3 In glucuronidation, the sugar acid, D-glucuronic acid (Figure 1), is coupled with a wide variety of compounds (see Chapter 2, Sections I and II) to form glycosides, the β-D-glucopyra-nosiduronic acids or, as termed throughout this book except where confusion could arise, the glucuronides.4
II. “Detoxication” Reactions
Conjugation is best known as a “detoxication” reaction. The validity of the term “detoxication” is discussed below, but the process can be considered as a progressive increase in polarity of a molecule. This increased polarity leads to its solubility in bile or urine and its consequent excretion from the body. The authority on detoxication, R. T. Williams, conveniently divided detoxication into two phases.5 Phase 1 consists of reactions such as oxidations, reductions, and hydrolyses whereby the molecule achieves a “handle”, a polar group such as -OH, -NH2, or -COOH. It is then able to enter Phase 2, where the “handle” takes part in conjugation. In Phase 2, conjugation with a highly polar molecule or ion such as glucuronic, sulfuric, or acetic acids usually ensures rapid excretion.5
A classical example, the detoxication of the drug phenacetin, is shown in Figure 2. It is quoted by Williams5 from the work of Smith and Williams.7
In Phase 1, the neutral lipid-soluble phenacetin is oxidatively deethylated to 4-acet-amidophenol. This compound is sparingly soluble in water, with a pKa of about 10, and is 0.25% dissociated at the body pH of 7.4.7 In Phase 2, the 4-acetamidophenol is conjugated with glucuronic acid to give the glucuronide, a strong acid of pKa 3.5, highly water soluble, and 99.99% dissociated at pH 7.4. 4-Acetamidophenyl glucuronide is readily excreted by the kidney. The body is, therefore, cleared of the drug by metabolism through Phases 1 and 2. A given compound (e.g., a phenol) may be ingested in a form already suitable for metabolism by Phase 2 enzymes. In that case, detoxication need not involve any Phase 1.
III. Distinctive Aspects of the Phase 2 Reactions of Detoxication
Phase 2 reactions are distinct from Phase 1 reactions. First, different sets of enzymes are involved in the two phases. Second, the products of Phase 1 reactions are still partially lipid soluble; many possess biological activity, either toxic or therapeutic. Thus 4-acetamidophenol, the Phase 1 metabolite of phenacetin noted above, is an active drug, the activity of administered phenacetin being largely due to this metabolite.7 Products of Phase 2 are usually, but not always, much less active, or quite inactive, biologically. This is due to two factors: (1) increased water solubility and decreased lipid solubility, allowing rapid removal from the body; (2) masking of biologically-active groups by superimposition of, or stereochemical hindrance by, the conjugating molecule. 4-Acetamidophenyl glucuronide in Figure 1 exhibits none of the antipyretic or analgesic properties of the administered phenacetin or its Phase 1 product.
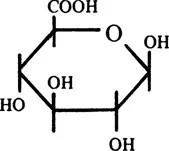
FIGURE 1. Structure of D-glucuronic acid.
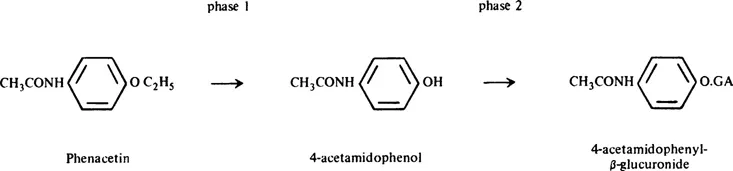
FIGURE 2. The detoxication of phenacetin.
Third, Phase 2 reactions are all synthetic reactions involving expenditure of energy. This energy can be applied by two methods to ensure conjugation. The first, and principal, method forms a “high-energy” endogenous molecule which donates the conjugating group, e.g., in formation of acetyl-coenzyme A, which later donates an acetyl group to the molecule, conjugating it as an acetate. Another example is the formation of UDPglucuronic acid, the donor of glucuronic acid.
The second method involves activation of the xenobiotic molecule to be conjugated. This is most common in conjugations with amino acids, as that of administered benzoic acid with glycine to form benzoylglycine (hippuric acid).
IV. The Concept of Detoxication
We must examine more closely the term “detoxication”. The use of the word “detoxication” presumably implies that toxicity of a molecule or ion to a particular species has been lessened or abolished. It would apply equally to molecules of endogenous and of exogenous origin, to conversion of ammonia to urea as much as to conversion of phenacetin to 4-acetamidophenyl glucuronide. Generally, the term is reserved for originally exogenous, usually lipid soluble, molecules. Such compounds, if not utiliz-able as fuel or building material by the species in question (i.e., if they are anutrients), will be metabolized by Phase 1, Phase 2, or both. The resulting metabolites will usually be more water soluble and less lipid soluble. They will, therefore, when once in blood or bile, remain there and not readily pass through lipid membranes into cells. From blood, they will be filtered by the kidney and pass down the tubules without reabsorption by the tubule cells, to appear almost quantitatively in the urine. The increased water-solubility and pKa values of some administered compounds and their glucuron-ides have been listed.6,8
We can now reconsider the concept of detoxication. Any detoxication of compounds X and Y that we see is merely an accompaniment of the metabolism of X and Y. X and Y enter the body and are oxidized, reduced, hydrolyzed, conjugated, or otherwise treated according to their chemical structure. Their structure alone determines what Phase they enter and by which of the host’s enzymes they are accepted. Their structure determines the structure of their metabolite, and so its solubility in water, its ease of excretion, and its toxicity. There is no guarantee that these metabolites will be less toxic than X or Y, and hence, no guarantee that X and Y will be truly detoxicated by the biphasic metabolism loosely termed “detoxication”.
This might be thought obvious. Yet much confusion, and several unfortunate incidents, have resulted from the assumption that th...