
eBook - ePub
Recent Advancement in Prodrugs
Kamal Shah, Durgesh Nandini Chauhan, Nagendra Singh Chauhan, Pradeep Mishra, Kamal Shah, Durgesh Nandini Chauhan, Nagendra Singh Chauhan, Pradeep Mishra
This is a test
Buch teilen
- 336 Seiten
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Recent Advancement in Prodrugs
Kamal Shah, Durgesh Nandini Chauhan, Nagendra Singh Chauhan, Pradeep Mishra, Kamal Shah, Durgesh Nandini Chauhan, Nagendra Singh Chauhan, Pradeep Mishra
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
Recent Advancement in Prodrugs
Drugs used as medicines have many limitations like low chemical stability, aqueous solubility, or oral absorption/bioavailability, rapid presystemic metabolism, toxicity, inadequate site specificity, or poor patient acceptance/compliance (unwanted adverse effects, unacceptable taste or odor, irritation or pain). Prodrugs design is an approach to overcome these limitations.
Key features
-
- Covers recent advancements in development of prodrugs
-
- Presents balanced synthesis and applications of prodrug chemistry
-
- Discusses broad spectrum of prodrug categories and outlines industrial applications
-
- Reviews prodrugs in cancer nanomedicine, its therapy and treatment
-
- Elucidates mathematical models to study the kinetics of prodrugs
This book covers recent advances in the design of prodrugs. It contains all the significant recent examples of prodrug chemistry developments and will aid academics and researchers seeking to generate new projects in the field.
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Recent Advancement in Prodrugs als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Recent Advancement in Prodrugs von Kamal Shah, Durgesh Nandini Chauhan, Nagendra Singh Chauhan, Pradeep Mishra, Kamal Shah, Durgesh Nandini Chauhan, Nagendra Singh Chauhan, Pradeep Mishra im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Physical Sciences & Clinical Chemistry. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
1
Recent Advancements in New Drug Design and Development of Prodrugs
Kamal Shah, Gaurav Krishna, Jeetendra Kumar Gupta, Durgesh Nandini Chauhan, Nagendra Singh Chauhan, and Pradeep Mishra
CONTENTS
1.1 Introduction
1.2 Prodrugs of Drugs Bearing Carboxylic Acids
1.3 Prodrugs of Alcohols and Phenols
1.4 Prodrugs of Amines
1.5 Prodrugs of Phosphonates, Phosphinates, and Phosphates
1.6 Peptides Bearing Prodrugs
1.7 Future Prospects
References
1.1 Introduction
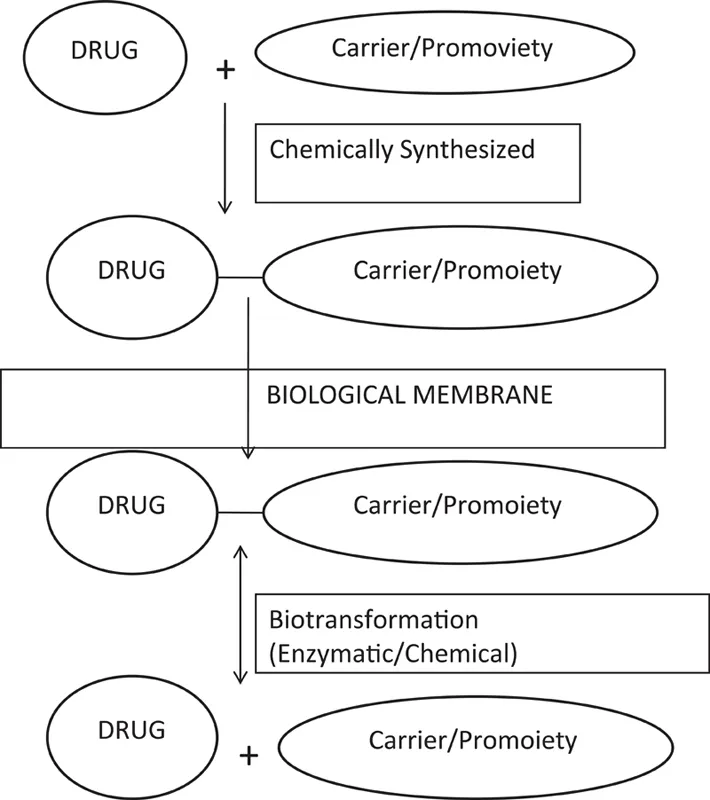
There are two major considerations in any drug design project. First of all, drug interacts with molecular target in the body and so it is important to choose the correct target for pharmacological activity. Secondly, a drug has to travel through the body in order to reach its target. Almost all drugs possess some undesirable side effects. The clinical efficiency can be enhanced by curtailing the unwanted side effects while accommodating the desired properties. A therapeutically promising drug may have some shortcomings so it cannot be used prominently (Remington and Gennaro 2000). A few of these shortcomings are poor aqueous solubility (corticosteroids), unpleasant taste (chloramphenicol), duration of action (estradiol, cytorabine, naloxone, testosterone), nonspecificity (carbidopa), poor bioavailability (carbencillin, ampicillin), gastric instability (erythromycin), etc. (Bhosle et al. 2006). These associated problems may be solved by different approaches. These are biological approaches that can be solved by the alteration in the route of administration or physical approach involves modification in the design of the dosage form such as controlled delivery of drugs and other is chemical approach, which is widely accepted and used mostly. The chemical approach may involve the design and development of novel drugs synthetically. These are synthesized which are essentially analogs of existing drugs and it can be termed as chemically derivatised prodrug. The term prodrug signifies that the molecule is not active or a chemical derivative of the drug. It becomes active after consumption. It generates or converts into active drug. The term prodrug was first coined by Albert (1958). He described prodrugs as compounds that undergo biotransformation before eliciting its activity. The chemical modification or transformation in prodrugs is done due to specific reasons. This process also referred as drug lamentation (Harper 1959) (Figure 1.1). It may be done to increase patient acceptability in terms of taste, solubility, bioavailability, stability and to decrease toxicity. These compounds are also known as bioreversible derivatives. The chemical transformation or modification results in formation of a covalent bond between drug and carrier. According to the carrier attached with prodrugs, Wermuth classified the prodrugs into two broad classes, i.e., carrier-linked prodrugs and bioprecursors. Carrier-linked prodrugs should have carrier that should release the active drug on in vivo transformation. Bioprecursors do not have any carrier attached; therefore, they are formed by molecular modification of active drugs. The conversion of prodrugs to active drug involves number of reactions (Sloan and Wasdo 2003). The type of reaction involved in this varies according to the presence of bonding or functional group arrangement (Stella et al. 1985). The design of prodrug requires comprehensive study of parent drug. The functional group present in drug may decide the linkage present in resultant prodrug. The preclinical studies of the designed prodrug should always be kept in mind, as the designed prodrug might alter the tissue absorption, bio distribution, its metabolism and kinetics (ADME). This alteration ultimately had effect over efficacy, potency and toxicity of resultant prodrug. The promoiety or carrier which is taken with drug should be such that it may have synergetic effect as in codrugs or mutual prodrugs. The phenomenon of codrug relates with co-administrating two drugs. The objectivity of such codrugs is to enhance pharmacological activity by delivering the drugs at desired site at the same time or masking the side effects of parent drug. The choice of promoiety may vary according to the problem identified in parent drug. A complete study of ADME (absorption, distribution, metabolism and excretion) is required during designing of prodrugs. The by-products formed from the prodrug should also be taken into consideration. The formed prodrug must rapidly biotransform into its active form whether chemically or enzymatically. The design of desired prodrug involves the use of functional groups present in parent drug. The functional groups that form covalent linkages may be alcohols, phenols, acids, amine, amide, secondary amines, imide, phosphates, etc. The prodrugs were designed with the aim that they may alter solubility, bioavailability, reduce toxicity, enhance acceptability, increase site specific delivery, increase duration of action, increase patient compliance, etc. This chapter is going to cover the recent examples of prodrugs with their possible chemistry and application. The prodrugs can be discussed according to functional group present in the parent structure.
1.2 Prodrugs of Drugs Bearing Carboxylic Acids
The carboxylic acid is the common functional group present in the drugs. The pKa value of such drugs generally lies between 3.5 and 4.5. These drugs when taken get ionized (deprotonated). The logD values of these drugs decline down the range of logD values of strongly absorbed drugs (logD > 0). This problem has been identified over the many years and one of the common solutions of this is esterification. The pervasiveness of esterase, peptidases and other enzymes in the organisms as well as the easily availability of alcohols and phenols containing codrugs result in development of number of prodrugs (Bundgaard 1985, Beaumont et al. 2003). The added advantages with carboxylic acids containing prodrugs are to obtain desirable hydrophilicity or in vivo activity.
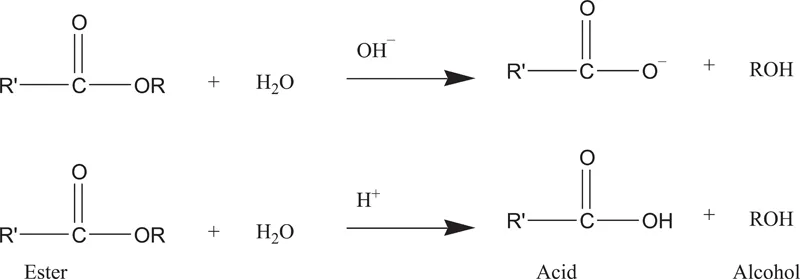
The general scheme for the hydrolysis of ester containing prodrugs are as follows (Figure 1.2).
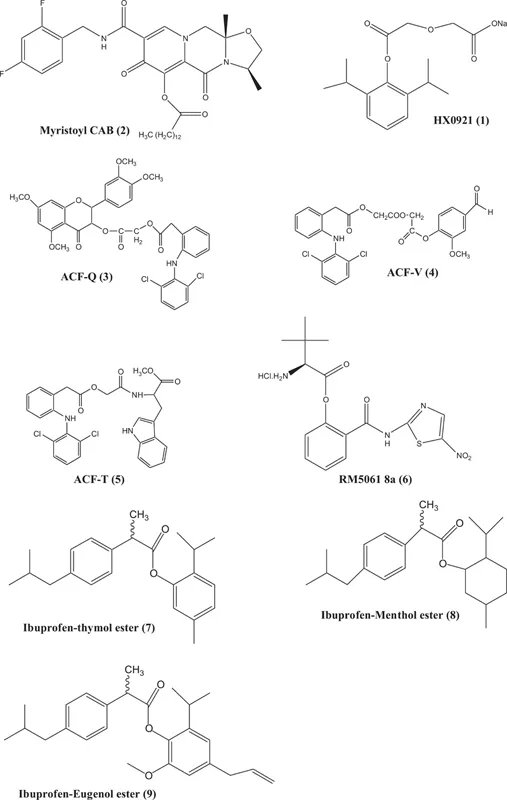
There are enormous examples of prodrugs having ester linkage in the literature. Some recent examples are as follows (Figure 1.3).
The prodrug of propofol synthesized by Zhang and group having ester linkage (Zhang et al. 2019). Propofol is a water insoluble drug. It is commonly given intra venous and have rapid onset of action. During this study, the synthesized prodrug of propofol, HX0921 (1) (sodium 2-(2-(2,6-diisopropylphenoxy)-2-oxoethoxy)acetate), showed good water-solubility. The pharmacological activities of this compound proof that it has short duration of action and onset also lowered. It was found that in prodrug form it hydrolyzed rapidly and showed its better activity. Zhou synthesized arbotegravir (CAB) nano-formulated prodrug (Myristoyl CAB) (2) to improve the properties of parent drug lipophilicity and its availability in circulation to have better antiretroviral profile (Zhou et al. 2018). The mutual prodrugs of aceclofenac with naturally occurring antioxidants, i.e., aceclofenac with quercetine (ACF-Q) (3), aceclofenac with vanillin (ACF-V) (4) and aceclofenac with L-tryptophan (ACF-T) (5). It was found these prodrugs were more lipophilic than aceclofenac with improved stability in acidic pH. The pharmacological activities, when compared with parent drug, were found to be better analgesic and anti-inflammatory agents with reduced ulcerogenicity (Rasheed et al. 2016). The amino acid esters of Nitazoxanide were verified for their animal activities. Its pharmacology, kinetics, and toxicity studies were carried. Among synthesized prodrugs, RM5061 8a (6) in rats showed sevenfold more blood concentration compared to previously reported drug (Stachulski et al. 2017). The synthesized prodrug had an excellent safety profile with least toxicity. Its bioavailability increased from 3% to 20%.
Redasani and Bari utilized natural phyto phenols like thymol, menthol and eugenol for acquiring the additive activity and lowers the gastric problems common with ibuprofen. The ester derivatives (Ibuprofen-thymol ester (7), Ibuprofen-menthol ester (8) and Ibuprofen-eugenol ester (9)) of ibuprofen were synthesized and evaluated (Redasani and Bari 2012). The prodrugs formed were found to be lipophilic and stable at acidic pH. They elicited improved anti-inflammatory activity. These prodrugs showed additive effect, the reason of its activity might be conjugation of ibuprofen to natural analgesics. The result of ulcer index showed that the synthesized prodrug had lower gastric ulceration than the parent drug. This is a clear example of masked carboxylic acid.
1.3 Prodrugs of Alcohols and Phenols
The drug-bearing alcohols and phenols groups are polar in nature. They often undergo phase II metabolism. The modification of these functional groups leads to form prodrugs it may lead to change the properties of parent drug. The chemical reactions like alkylation, acylation or reduction would decrease the polarity, increases the lipophilicity so it increases membrane permeability however phosphorylation result in solubility enhancement. Many drugs that are sufficiently absorbed through gastro intestinal tract (GIT) have lower systemic concentration that is due to the first-pass metabolism. This prodrug approach circumvents the high first pass-metabolism and consequently increases the systemic concentration. The best possible way is esterification of the drugs having hydroxyl or phenolic group(s). Esterification may lead to form prodrugs of significant lipophilicity and have better in vivo lability (Longcope et al. 1985). Some of the recent examples are as follows (Figure 1.4).
The prodrugs of haloperidol were synthesized and evaluated for their hydrolysis in simulated environment corresponding to liver and small intestine. Haloperidol pentanoate (10) and Haloperidol hexanoate (11) showed high metabolic activation rates in the formulated esterprodrugs (Takahashi et al. 2019). Marinelli and his team synthesized 23 compounds and screened the antimicrobial activity. Among the synthesized compounds, compounds WSCP18 and 19 (12 and 13) showed good antimicrobial activity (Marinelli et al. 2019). These were found to be more stable and lipophilic. The novel benzoic acid–based xanthine derivatives synthesized among that compound 3e (BA-XN) (14) sustained the antidiabetic effect for 48 h and result in improved glucose tolerance and found norm...