![]()
1
Introduction
Anthony J. Hickey and Sandro R.P. da Rocha
A number of outstanding texts on foundational elements of the topics discussed in this book exist, and the reader is encouraged to familiarize themselves with these materials, as they describe basic principles (Finlay, 2001), specific (Purewal and Grant, 1997, Srichana, 2016 and Zeng et al, 2000) and general dosage forms (Colombo et al., 2013, Hickey, 2007, Newman, 2009, Smyth and Hickey, 2011), and analytical methods (Tougas et al., 2013).
The advances in pharmaceutical inhalation aerosol technology occurring since the turn of the millennium have increased the potential of pulmonary drug delivery substantially. While some of the new developments had their origins in earlier work, we have seen the appearance of new propellants and new regulations considering the phase out of what we still consider new propellants, new dry powder inhalers, nebulizers, and a new category of product, soft mist inhalers.
In parallel with these new products, the breadth of application has increased to include the treatment of chronic obstructive pulmonary disease, a range of infectious diseases, diabetes, idiopathic pulmonary fibrosis, and pulmonary arterial hypertension. Pre-clinical studies and clinical trials covering yet a range of other potential applications of orally inhaled products include the use of a broader range of biologics and also nanomaterials that may help further advance the pulmonary drug delivery market.
Successful aerosol therapy has given research and development a boost, and the prospects of even greater opportunities for disease management is emerging from patient compliance, adherence tools, and new classes of drugs for local and systemic delivery through the lungs.
This text is focused on the active pharmaceutical ingredient, formulation development, device design, process and product engineering, and analytical methods to assess critical quality attributes underpinning safe and efficacious dosage forms.
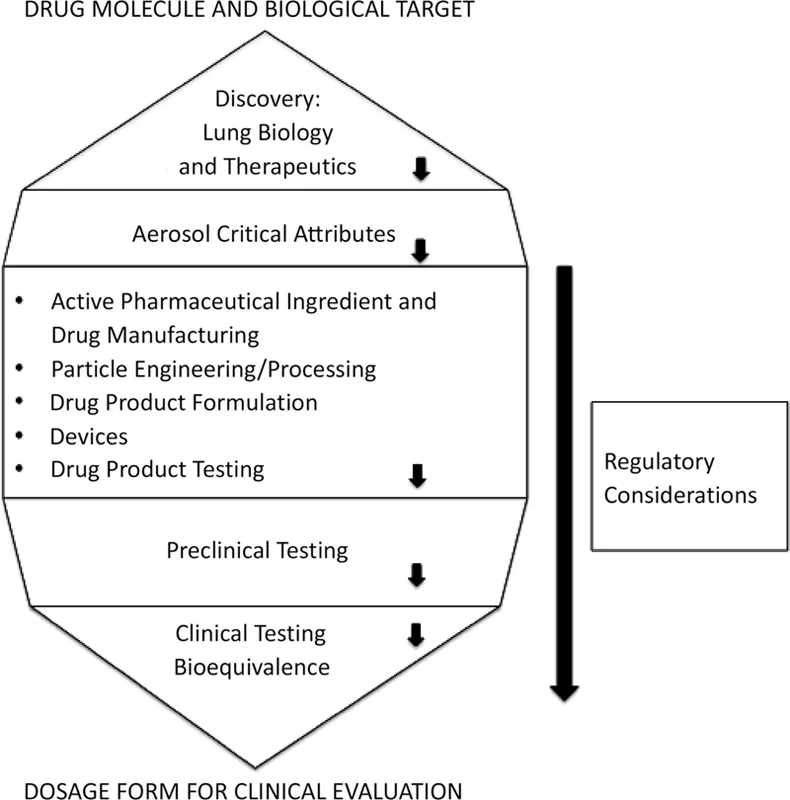
Figure 1.1 depicts the sequence in which these topics will be presented, which follows the product development pathway. The conclusion of the volume is a discussion of bioequivalence testing and the interface between the dosage form and the patient. This reflects the point at which design and engineering controls, which are embedded in a regulated environment of quality by design, give way to biological factors.
It is intended that the materials covered in subsequent sections familiarize the reader with the underlying science and engineering associated with the design and characterization of complex dosage forms required to deliver orally inhaled aerosols. The platform of knowledge will be useful in considering options for specific applications and is a point from which to launch new technologies that will frame future developments in the field as described in a companion text (Hickey and Mansour, in press).
FIGURE 1.1 Product development themes in pharmaceutical inhalation aerosol technology.
REFERENCES
Colombo P, Traini D, Buttini F. Inhaled Drug Delivery: Techniques and Products. New York: Wiley-Blackwell; 2013.
Finlay W. The Mechanics of Inhaled Pharmaceutical Aerosols: An Introduction. New York: Academic Press; 2001.
Hickey A. Inhalation Aerosols, Physical and Biological Basis for Therapy. 2nd ed. New York: Informa Healthcare; 2007.
Hickey A J, Mansour H H, Eds. Inhalation Aerosols, Physical and Biological Basis for Therapy, Third Edition. Boca Raton, FL: CRC Press; in press.
Newman S. Respiratory Drug Delivery: Essential Theory and Practice. Richmond, VA: RDD Online; 2009.
Purewal T, Grant D. Metered Dose Inhaler Technology. Boca Raton, FL: CRC Press; 1997.
Smyth H, Hickey A. Controlled Pulmonary Drug Delivery. New York: Springer; 2011.
Srichana T. Dry Powder Inhalers: Formulation, Device and Characterization. Hauppauge, NJ: Nova Science Publishers; 2016.
Tougas T, Mitchell J, Lyapustina S, Eds. Good Cascade Impactor Practices, AIM and EDA for Orally Inhaled Products. New York: Springer; 2013.
Zeng X, Martin G, Marriott C. Particulate Interactions in Dry Powder Formulations for Inhalation. New York: CRC Press; 2000.
![]()
Section I
Discovery
![]()
2
Physiology of the Airways
Anthony J. Hickey and David C. Thompson
CONTENTS
2.1 Introduction
2.2 Anatomy of the Airways
2.2.1 Structure
2.2.1.1 Epithelium
2.2.1.2 Smooth Muscle Cells
2.2.1.3 Gland Cells
2.2.1.4 Nerves
2.2.1.5 Defensive Cells
2.2.1.6 Blood Supply
2.2.2 Zones of the Airways
2.2.2.1 Conducting Zone
2.2.2.2 Respiratory Zone
2.3 Function of the Airways
2.3.1 Gas Exchange
2.3.2 Acid-Base Balance
2.3.3 Endocrine
2.3.4 Metabolism
2.4 Evaluation of Airway Physiology and Function
2.4.1 Measures of Pulmonary Volumes
2.4.2 Measures of Airway Caliber
2.4.2.1 Peak Flow Measurements
2.4.2.2 Forced Expiratory Flow Measurements
2.4.2.3 Airways Resistance and Dynamic Lung Compliance
2.5 Aerosol Deposition and Airway Physiology
2.6 Conclusion
References
2.1 Introduction
The airways represent a unique organ system in the body, their structure allowing air to come into close contact with blood, is one of the principal adaptions permitting the existence of terrestrial life. This adaptation also makes the airways a useful route of administration of drugs in the inhaled or aerosol form. This chapter provides an overview of the physiology of the airways excluding that of the nasopharyngeal regions of the airways. Aspects considered relevant to the practical and theoretical application of inhaled substances are emphasized.
2.2 Anatomy of the Airways
The airways (constituting the lungs) may be viewed as a series of dividing passageways originating at the trachea and terminating at the alveolar sac. In the context of aerosol design and delivery, such a “static” overview represents a satisfactorily simple model. However, many factors beyond the anatomy of the airways are relevant to the therapeutic use of aerosols.
2.2.1 Structure
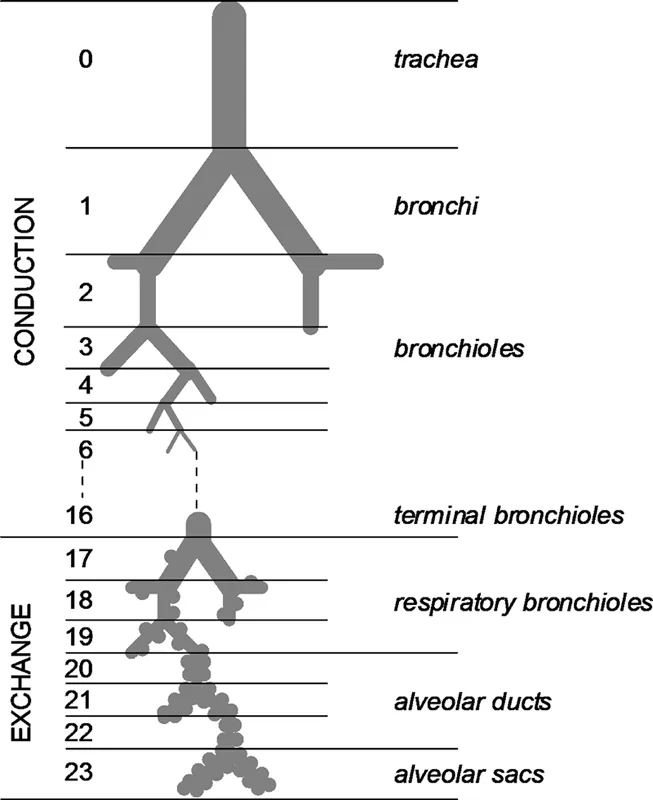
The airways are often described as the pulmonary tree in that their overall form resembles a tree. The tree trunk is analogous to the trachea of the airways that bifurcates to form main bronchi. These divide to form smaller bronchi that lead to individual lung lobes: three lobes on the right side and two on the left side. Inside each lobe, the bronchi undergo further divisions to form new generations of smaller caliber airways: the bronchioles. This process continues through the terminal bronchioles (the smallest airway not involved with an alveolus), the respiratory bronchioles (which exhibit alveoli protruding from their walls), alveolar ducts, and terminates with the alveolar sacs. In the classic model of the airways, as described by Weibel (1963), each airway divides to form two smaller “daughter” airways (Figure 2.1), and, as a result, the number of airways at each generation is double that of the previous generation. The model proposes the existence of 24 airway generations in total, with the trachea being generation 0 and the alveolar sacs being generation 23.
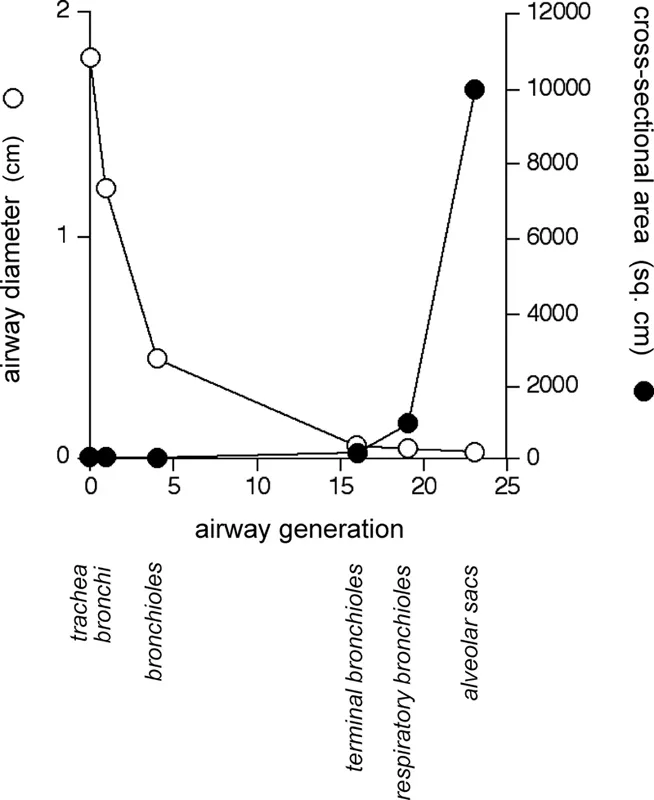
In passing from the trachea to the alveolar sac, two physical changes occur in the airways that are important in influencing airway function. Firstly, the airway caliber decreases with increasing generations, for example, tracheal diameter ≈ 1.8 cm versus alveolar diameter ≈ 0.04 cm (Figure 2.2). This permits adequate penetration of air to the lower airways for a given expansion of the lungs. Secondly, the surface area of the airways increases with each generation to the extent that the total area at the level of the human alveolus is in the order of 140 m2 (Gehr et al., 1978). The alveolus is the principal site of gas exchange in the airways, a function compatible with the increased surface area that promotes extensive and efficient diffusional gas exchange between the alveolar space and the blood in alveolar capillaries (vide infra). The relatively small change in cross-sectional area that occurs over the 19 generations of airways between the trachea and the terminal bronchiole (from 2.5 cm2 to 180 cm2) (Bouhuys, 1974) fosters the rapid, bulk flow of inspired air down to the terminal bronchiole. By contrast, the cross-sectional area increases greatly in the four generations between the terminal bronchiole and the alveolar sac (from 180 cm2 to 10,000 cm2) (Bouhuys, 1974), which results in a significant decrease in the velocity of airflow to the extent that the flow velocity fails to exceed that of diffusing oxygen molecules (Weibel, 1984). Accordingly, diffusion assumes a greater role in determining the movement of gases in these peripheral airways.
FIGURE 2.1 Model of airway. (With kind permission from Taylor & Francis: Morphometry of the Human Lung, Berlin, Germany, Springer-Verlag, 1963, Weibel, E.)
FIGURE 2.2 Graph of airway diameter and cross-sectional are as a function of airway generation.
The various levels of the airways may be categorized functionally as being either conducting or respiratory airways. Those airways not participating in gas exchange constitute the conducting zone of the airways and extend from the trachea to the terminal bronchioles. This region is the principal site of airway obstruction in obstructive lung diseases, such as asthma. The respiratory zone includes airways involved with gas exchange and comprises respiratory bronchioles, alveolar ducts, and alveolar sacs. As such, conducting and respiratory zones of the airways may be distinguished simply by the absence or presence of alveolar pockets (which confer gas exchange function). Regions within each zone may be classified further on a histological basis. For example, the contribution of cartilage to the airway wall is one means of differentiating the trachea from bronchi and bronchioles because cartilage exists as incomplete rings in the trachea, regresses to irregularly shaped plates in bronchi, and is absent from bronchioles. Also, respiratory bronchioles may be discriminated from terminal bronchioles by the pres...