
eBook - ePub
Perspectives in Behavioral Medicine
Eating Regulation and Discontrol
Herbert Weiner,, Andrew S. Baum,, Herbert Weiner,, Andrew S. Baum,
This is a test
Buch teilen
- 248 Seiten
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Perspectives in Behavioral Medicine
Eating Regulation and Discontrol
Herbert Weiner,, Andrew S. Baum,, Herbert Weiner,, Andrew S. Baum,
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
First published in 1987. Routledge is an imprint of Taylor & Francis, an informa company.
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Perspectives in Behavioral Medicine als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Perspectives in Behavioral Medicine von Herbert Weiner,, Andrew S. Baum,, Herbert Weiner,, Andrew S. Baum, im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Psicología & Historia y teoría en psicología. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
1
REGULATION OF GASTRIC SECRETION—NEURAL MECHANISMS
The importance of neural mechanisms in the regulation of gastric secretion has passed through at least three cycles. In Pavlov’s time, nerves were considered to be of primary importance as demonstrated in conscious dogs with esophagostomies (sham feeding) and in the comparison of the responses of vagally innervated (Pavlov) gastric fundic pouches to those of vagally denervated (Heidenhain) pouches. Then came the pioneering experiments of Bayliss and Starling and Edkins in the role of hormonal regulation, culminating in the purification and isolation of gastrin by Gregory and his associates. Hormones became the dominant factors, including hormones released by nerve stimulation (e.g., gastrin). We are now in a third phase in which the same substance may play a role in the regulation of gastric secretion as a classical hormone, a neurotransmitter, a neuromodulator, and a paracrine messenger.
I have reviewed the subject on several occasions in the past (Brooks, 1965, 1967, 1968, 1975, 1977, 1981). The number of publications on the subject has increased substantially recently, as indicated by the approximately 20 related abstracts submitted for presentation during Digestive Disease Week in 1985. In this review, I will consider nervous regulation of gastric exocrine and endocrine secretion by nerves within the wall of the stomach, by extrinsic nerves, and by the brain.
INTRAMURAL NERVOUS CONTROL
Structure
The structural basis for the innervation of secretory cells in the stomach is still incompletely understood. Light microscopic studies show bare nerve endings in close contact with parietal cells in rabbits (Hanker, Tapper, & Ambrose, 1977). In the cat, nerve endings reached only the basal half of the fundic glands (Kyosola, Veyola, & Richardt, 1975). More recently, electron micrographs showed no nerve axons or varicosities closer than 100 nM to parietal cells in the opossum (Seelig, Schlusselberg, & Woodward, 1983); and similar findings have been reported for rat mucosal epithelial cells (Crocket, Doyle, & Joffee, 1981) and rhesus monkey parietal cells (Lechago & Barajas, 1976). The latter were stained for acetylcholinesterase. It is interesting that in salivary glands an electrical intracellular response to single parasympathetic nerve impulses was seen only when the gap between nerve endings and secretory cells was about 20nM (Garrett, 1974). Cholinergic nerve endings have also been demonstrated in contact with gastrin cells (Lechago & Barajas, 1981).
Recent immunohistochemical studies have identified regulatory peptides such as substance P, vasoactive intestinal polypeptide (VIP), enkephalins, and the gastrin-cholecystokinin (CCK) peptides near gland cells. Cell bodies in the myenteric plexus of the stomach contained immunoreactive substance P, VIP, and enkephalins (Schultzberg et al., 1980). The submucosal plexus is poorly developed in the stomach. Gastrin-releasing polypeptide is reported to be present in nerve endings in the pyloric antrum. Nerve endings with vesicles consistent with neuropeptides have been found within 200–300 nm of gastrin cells (Lechago & Barajas, 1981).
Cellular Physiology of H+ Secretion
The development of relatively pure parietal and chief cell preparations has made it possible to examine the role of neurotransmitters acting on gastric secretory cells. Acetylcholine stimulated the accumulation of acid in the canaliculi of isolated parietal cells. The effect was blocked by atropine (Soll, 1980). Saturable, temperature-dependent binding of [3H]QNB(Quinuclidinyl benzilate—an anticholinergic), to rat parietal cells in vitro has been demonstrated, which is consistent with the presence of cholinergic receptors (Ecknauer, Thompson, Johnson, & Rosenfeld, 1980). The second messenger in stimulus-secretion coupling appears to be calcium, rather than cyclic AMP (Soll, 1981).
The accumulation of H+ in parietal cells in response to acetylcholine, histamine, or gastrin was blocked by H2 antagonists such as cimetidine (Soll, 1981). There are two hypotheses to account for this: one, proposed by Soll, is based on potentiation between receptors for the three stimulants (Soll, 1982); the other proposes that acetylcholine and gastrin release histamine (Lorenz, Mohri, Reimann, Troidl, Rohde, & Barth, 1980). In non-rodent mammals, the mucosal source of histamine appears to be a mastlike cell (Lorenz, Thon, Barth, Neugebauer, Reimann, & Kusche, 1983). Histamine release in response to nerve stimulation has yet to be established. Figure 1.1 shows the interactions between receptors on the parietal cell, while Figure 1.2 illustrates the role of histamine as a common mediator.
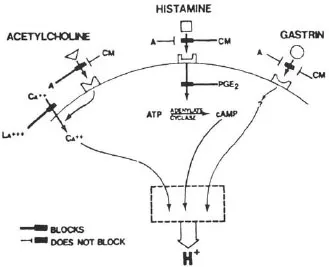
FIGURE 1.1 Receptors on the parietal cell mediating stimulation of acid secretion. Cimetidine (CM), a histamine 2 antagonist, blocks stimulation by histamine, acetylcholine, and gastrin; atropine (A) blocks only the action of acetylcholine. The second messenger for acetylcholine and gastrin is probably calcium, while that for histamine is cyclic AMP. Prostaglandin E2 blocks the activation of cAMP by histamine and thereby inhibits acid secretion. Calcium is able to stimulate acid secretion by penetrating the parietal cell membrane. This is blocked by lanthanum. (From Soll, A. H. (1981). Physiology of isolated canine parietal cells: Receptors and effectors regulating function. In L. R. Johnson (Ed.), Physiology of the gastrointestinal tract, pp. 673–691. New York: Raven Press.
A calcium-calmodulin complex in the parietal cell may determine levels of calcium-dependent protein kinases which are thought to influence the availability of potassium ions for the exchange of H+ for K+ at the canalicular membrane, powered by the H+ −K+ ATPase or proton pump (Walker, Vinik, Heldsinger, & Kaveh, 1983).
Similar results have been obtained with isolated fundic glands (Berglindh, 1977). It is interesting that glands from human stomachs after vagotomy accumulated H+ at a reduced sensitivity to both histamine and carbachol (Leth, Elander, Fellenius, Haglund, & Olbe, 1981).
Nervous Stimulation of Acid Secretion in the Isolated Stomach
Electrical field stimulation of the isolated mouse stomach stimulated acid secretion. It was blocked by tetrodotoxin and reduced by atropine and hexamethonium, a ganglionic cholinergic blocker (Angus & Black, 1978). Mucosal preparations from rat stomach responded similarly (Baird & Main, 1978). These results are consistent with stimulation of acid secretion by cholinergic neurons in the myenteric plexus.
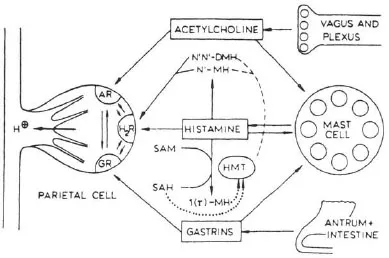
FIGURE 1.2 Stimulants to acid secretion by the parietal cell. Histamine released from mast cells is accorded the central role in this model of the control of acid secretion. Both acetylcholine and gastrin release histamine from mast cells as well as occupying receptors on the parietal cell. The metabolism of histamine is indicated. AR = acetylcholine receptor; H2 R = H2 receptor; GR = gastrin receptor; N1N1 − DMH = N2N2 − methyl histamine; N1 − MH = N2 methyl histamine; SAM = S-adenosylhomocysteine; 1 (r) − MH = r methyl histamine; HMT = histamine methyl transferase acting as agonist or releaser, → acting as an inhibitor → acting either as an activator or inhibitor depending on the condition. (From Lorenz, W., Troidl, H., & Barth, H. (1975). Stimulus-secretion coupling in the human and canine stomach: Role of histamine. In R. M. Case & H. Goebel (Eds.), Stimulus secretion coupling in the gastrointestinal tract (p. 179). Lancaster, PA: MTP Press Ltd.
Pepsinogen and Intrincis Factor Secretion
Rabbit gastric mucosa in organ culture responded to acetylcholine by increasing secretion of pepsinogen and intrinsic factor. The effect was blocked by atropine (Kapadia & Donaldson, 1978). In the isolated fundic gland preparation from rabbits, both acetylcholine and the β-adrenergic agonist isoproterenol stimulated the release of pepsinogen in a dose-related manner. Isoproterenol was without effect on gastric acid secretion (Koelz, Hersey, Sachs, & Chew, 1982). The former’s effect was blocked by atropine and the latter by propranolol. Cholinergic binding sites on chief cells in isolated gastric glands have been demonstrated with QNB (Culp, Wolosin, Soll, & Forte, 1983). Similar results were obtained with canine chief cells in primary monolayer culture (Sanders, Amirian, Ayalon, & Soll, 1983).
Alkaline Secretion from Surface Epithelial Cells of the Stomach
Amphibian gastric fundic mucosa secrete bicarbonate actively in vitro in response to carbachol (Flemstrom, 1977). This appears to be the result, in part, of a chloride-bicarbonate exchange at the luminal membrane (Flemstrom & Garner, 1982).
Cholinergic and Adrenergic Control of Secretion in Vivo
Cholinergic and adrenergic transmitters can be given by close intra-arterial or by intravenous injection to determine the pharmacologic actions of these agents. On the other hand, anticholinergics or antiadrenergic drugs can be given by a variety of routes and the extent to which they inhibit nerve-mediated secretion determined. Stable analogues of acetylcholine are potent stimulants of acid secretion in animals but not in man. Beta-adrenergic agonists given intravenously stimulate acid secretion, probably secondary to the release of gastrin (Geumei, Issa, El-Gendi, & Abd-El-Samie, 1969).
The secretion of a bicarbonate-rich fluid with an otherwise similar composition to an ultrafiltrate of plasma can be obtained in dogs after the close intra-arterial injection of acetylcholine (Altimirano, 1963). Mucus secretion was also stimulated (Flemstrom, 1977).
Gastrin and Somatostatin Secretion
Antral mucosa from rats in organ culture released immunoreactive gastrin into the medium in response to carbachol in a dose-related manner (Harty & McGuigan, 1980). It was blocked by atropine. The incorporation of 3H-tryptophane into gastrin also increased in response to carbachol (Harty & McGuigan, 1980). At the same time, the release of somatostatin was reduced by carbachol. The presence of antibodies to somatostatin increased the gastrin released in response to carbachol by 69% (Wolfe, Reel, & McGuigan, 1984). Somatostatin reduced carbachol-induced release of gastrin (Harty, Maico, & McGuigan, 1981). Both norepinephrine and isoproterenol released gastrin and inhibited the release of somatostatin, suggesting parallel cholinergic and β-adrenergic control of gastrin and somatostatin release (Spindel, Harty, & McGuigan, 1984).
Gastrin-releasing peptide (GRP) released gastrin in this preparation. GRP appears to mediate gastrin release in response to β-adrenergic stimulation (Wolfe, Reel, Short, & McGuigan. 1984). Gamma-aminobutyric acid (GABA) released gastrin apparently by releasing acetylcholine from nerve endings (Harty & Franklin, 1983). Adenosine, on the other hand, inhibited the release of gastrin in response to carbachol (Harty & Franklin, 1984). However, carbachol had no effect on gastrin secretion by isolated canine antral mucosal cell cultures (Sugano, Park, Soll, & Yamada, 1984).
Use of the whole stomach in vitro permits a clear distinction between secretion into the vascular compartment and release into the lumen. Gastrin secretion by isolated perfused rat stomachs was increased by methacholine. Seventy-nine percent was secreted into the venous effluent and 21 % into the lumen. The effect was blocked by atropine. The secretion of somatostatin was reduced in a reciprocal fashion (Saffouri, Weir, Bitar, & Makhlouf, 1980). Bombesin released gastrin and somatostatin into the venous effluent. Figure 1.3 shows a proposed model for the nervous control of gastrin and somatostatin secretion (Du Val, Saffouri, Weir, Walsh, Arimura, & Mahklouf, 1981). There is controversy over the role of ganglionic receptors in the cholinergic control of gastrin and somatostatin secretion. One group of investigators reported that nicotinic receptor stimulation caused the secretion of both gastrin and somatostatin. Atropine completely blocked only the latter (Schubert & Makhlouf, 1982). An antiserum to bombesin reduced gastrin release by two thirds in response to a nicotine ganglionic agonist (Schubert, Walsh, & Makhlouf, 1983). The other group found that hexamethonium, a nicotinic receptor blocker, had no effect on bombesininduced gastrin release but blocked the release of somatostatin. They concluded that no ganglionic receptors were involved in carbacholine-induced release of gastrin or the inhibition of somatostatin release (Martindale, Kauffman, Levin, Walsh, & Yamada, 1982).
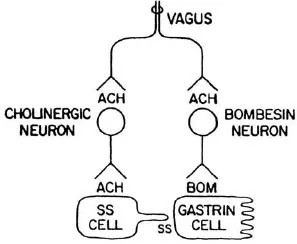
FIGURE 1.3 Model of the neurohumoral control of gastrin secretion. Vagal efferent impulses release acetylcholine which transmits impulses to both a cholinergic inhibitory neuron to somatostatin-secreting cells and a bombesin-like neuron which stimulates gastrin secretion. Somatostatin paracrine secretion inhibits gastrin release. Therefore, vagal stimulation stimulates ga...