
eBook - ePub
Environmental Sampling and Analysis
Lab Manual
Maria Csuros
This is a test
Buch teilen
- 400 Seiten
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Environmental Sampling and Analysis
Lab Manual
Maria Csuros
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
This manual covers the latest laboratory techniques, state-of-the-art instrumentation, laboratory safety, and quality assurance and quality control requirements. In addition to complete coverage of laboratory techniques, it also provides an introduction to the inorganic nonmetallic constituents in environmental samples, their chemistry, and their control by regulations and standards.
Environmental Sampling and Analysis Laboratory Manual is perfect for college and graduate students learning laboratory practices, as well as consultants and regulators who make evaluations and quality control decisions. Anyone performing laboratory procedures in an environmental lab will appreciate this unique and valuable text.
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Environmental Sampling and Analysis als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Environmental Sampling and Analysis von Maria Csuros im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Physical Sciences & Chemistry. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
Chapter 1
INTRODUCTION
TO CHEMICAL ANALYSIS
TO CHEMICAL ANALYSIS
1.1 THE NATURE OF ANALYTICAL CHEMISTRY
Chemical analysis of a sample of matter is the determination of the chemical composition of that sample. Chemical analysis consists of a set of chemistry laboratory operations designed to reveal the chemical makeup of a material. Analytical chemistry is a branch of chemistry dealing with the separation and analysis of chemical substances.
Analytical chemistry can be divided into two major categories: qualitative and quantitative analytical chemistry. Qualitative analysis is concerned with what is present, quantitative analysis with how much. Analysis procedures can be additionally classified into wet chemistry procedures and instrumentation procedures (see Figure 1.1). Wet techniques are those that employ chemical reactions and classical reaction stoichiometry for results.
Wet chemistry procedures give excellent accuracy, but their major disadvantages are the time requirement and subsequent tedium. Instrumental or “modern” techniques give speed and offer a much greater scope and practicality to the analysis. In addition, the much lower detection limits of the instrumentation analytical methods make possible the quantitation of the minor constituents in a substance. Although a number of purely instrumental methods of analysis have been developed, chemical methods are still vital and widely used, for several reasons. Many instruments require extensive calibration for each type of sample to be analyzed, whereas chemical methods can be quickly adapted to analyzing new types of samples. Few instrumental methods can match the accuracy and precision of chemical methods. For example, instrumental methods are excellent for the analysis of trace constituents, but their accuracy with respect to major constituents is greatly deficient to that achieved by wet chemistry methods. By proper selection, both instrumentation and wet chemistry methods are used equally in laboratories, and they supplement each other. On the other hand, knowledge of chemical methods provides the background to understanding instrumental analysis and analytical problems.
1.2 GENERAL DIRECTION OF CHEMICAL ANALYSIS
The results of a chemical analysis could affect important decisions, such as suitability of a material for an intended purpose, the quality of the environment, the health of individuals, the freedom of a prisoner on trial, etc. Stringent requirements in a chemical laboratory are the basis of reliable and comparable data from a chemical analysis.
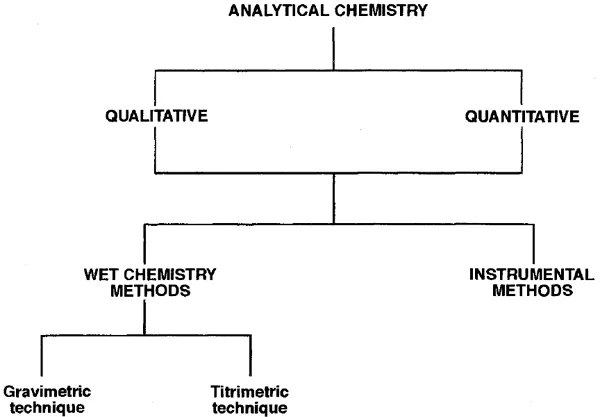
Figure 1.1
Analytical chemistry can be classified as either qualitative or quantitative. All analytical procedures can be additionally classfied into those that are wet procedures and those that are instrumental procedures. Wet chemical methods are divided into gravimetric technique (based on weight) and titrimetric technique (based on volume).
Analytical chemistry can be classified as either qualitative or quantitative. All analytical procedures can be additionally classfied into those that are wet procedures and those that are instrumental procedures. Wet chemical methods are divided into gravimetric technique (based on weight) and titrimetric technique (based on volume).
1.2.1 The Responsibility and Psychology of the Analyst
“Chemical analysts and chemical technicians are professionals in every sense of the word. They should look, think, and act as professionals are expected to do. They are entitled to all the privileges of professionals and at the same time are expected to shoulder the responsibilities of professionals.” A true analyst, or analytical chemist, has several characteristics. He or she has a knowledge of the methods and instruments used for analysis, and understands the principles of the analysis. Laboratory analysts should have a chemistry background adequate to understand and correctly apply all of the laboratory rules and to evaluate and interpret the results of their analysis. They have to know how to plan and organize laboratory work so the time is used efficiently. Thus, a laboratory technician should be a skilled, well-trained chemist — in sharp contrast with the so-called “determinators” who simply twist the dials of an instrument or follow “cookbook” analytical procedures.
The analyst should be familiar with the test methods described in the laboratory standard operation procedures (SOP) as well as the required quality assurance-quality control (QA/QC) applications as stated in the laboratory comprehensive QA/QC manual. The analyst should run the test by following the approved method and incorporate all QC checks according to the laboratory comprehensive QA/QC program. He or she should be able to recognize problems, initiate and conduct corrective actions, and keep all doc umentation related to the analysis clean and in order to be ready for inspection at any time. The analyst should be able to protect and defend all of the raw data as well as the reported results.
Laboratory personnel must understand the potential hazards in the laboratory and then become familiar with the precautions and safety rules to be followed in laboratory work. Accidents can be minimized by recognizing their causes and by being alert. They should know basic emergency first-aid treatment for minor injuries and also the location and use of emergency equipment. Laboratory safety procedures are discussed in Chapter 2.
1.2.2 Cleanliness in the Laboratory
Good analysts should be scrupulously clean and neat. An orderly laboratory bench is not prone to mix up samples, use wrong reagents, spill solutions, and break glassware. Much time can be wasted in searching for a small item in a jumble of glassware or in finding a certain reagent bottle that has been misplaced on the shelf. Cleanliness also includes laboratory equipment, ovens, hot plates, hoods, balances, sinks, floors, shelves, cabinets and laboratory clothes.
The result of a chemical analysis depends on clean glassware. Glassware that looks clean may or may not be clean for chemical analysis. Surfaces on which no visible dirt appears are often contaminated by minute quantities of a substance that interfere with the analysis and give false results. The analyst must carefully read the method and consider any special recommendation for glassware washing procedures.
1.2.3 Recording Analytical Data
Data obtained in the analytical laboratory should be recorded. The record should be complete, understandable, and easy to find.
Laboratory notebook — The key to successful work in the analytical laboratory is an orderly notebook with well-organized data. All documentation related to the raw data and the reported results should be in a bound form, easy to identify, and ready for inspection at any time. A bound notebook is preferred to a loose-leaf one. The size of the notebook should be adequate and comfortable to use. Preferably, it should fit easily around the balance and working table, leaving space for glassware, reagents, etc. necessary for the analysis. Larger notebooks, especially when opened, tend to get in the way and can cause spills and other problems. The first two pages should be reserved for an index, which can list the page numbers and the subject. If the pages of the notebook are not numbered, the analyst must number each page in the upper corner.
The title heading should be used to identify and date data on each page. All raw data and any observations should be recorded directly in the notebook at the time the work is performed. Especially forbidden is the recording of data on loose paper with the idea of copying it into the notebook later. All data should be written in ink to avoid smearing and erasures. Nothing should be erased; anything not needed should be crossed out with a single line, followed by an explanatory note, initialed, and dated.
Working sheets — Analytical raw data with related QC work and calculated final results may be documented in specified worksheets. The worksheets are designed for each type of analytical performance, and contain the information relating the sample, such as ID number (identification number), sample type, source, analytical method or method number with reference, method detection limit (MDL), specification of instrument used, ana–lytical and QC data, calculated values and proper units. Shortly, the worksheet contains all information necessary for validation of the analytical process, and the reported values. Figures 1.2, 1.3, and 1.4 are examples of laboratory worksheets. The designs of these documentation forms are just examples; each laboratory has its own patterns as approved in its QA/QC programs. As with all documentation, these sheets should be stored in bound form per analytical groups and parameters with document title, date start and end, identification number start and end. Strip charts, documented AA calibration curves, and raw data should be stored in file boxes and identified as mentioned above. Additional examples for working paper formats can be found in Appendix C.
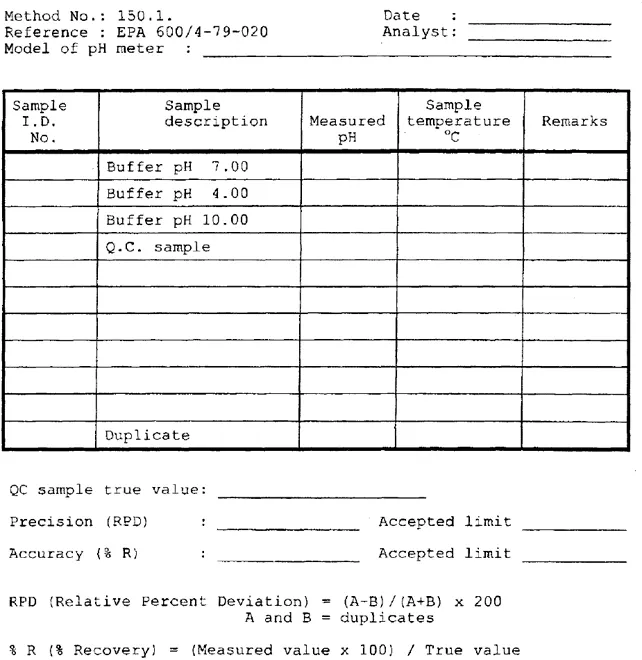
Figure 1.2
Working paper for pH measurement.
Working paper for pH measurement.
1.2.4 Planning Laboratory Work
It is essential to plan laboratory work ahead of time, so as to use the laboratory time efficiently and to avoid delays in using equipment that must be shared with other analysts. The following questions should be asked before the actual preparation for analysis: which kind of samples will be analyzed, how many samples analyzed, what is the source of the samples, which tests should be performed, which method should be used, which preservative was used, which pretreatment is necessary, etc.

Figure 1.3
Working paper for titrimetric analysis.
Working paper for titrimetric analysis.
The following list contains the duties of the analyst from the choosing of proper samples for the particular analysis through the actual analytical performance, QC checks, documentation, calculations, recognition and correction of problems, and giving accurate, defendable results.
• The analyst should be familiar with the method and the method should be followed as described in the laboratory standard operation procedure (SOP).
• If the sample needs pretreatment prior the actual analysis, such as drying, filtration, distillation, digestion, extraction, etc., start as soon as possible, because these are time-consuming procedures. If sample preparation is the duty of another laboratory department, collect all of the information needed for calculation of the final result (original volume, weight, final volume after treatment, etc.).
• When samples are stored in a refrigerator, remove them in time to allow the samples to warm up to room temperature prior to starting the analysis.
• Carefully check sample label for identification and select samples according to the appropriate bottle type and preservation for the analysis.
• Collect all standards and reagents needed and che...