Tumor Resistance Mechanisms to Inhibitors Targeting the Epidermal Growth Factor Receptor– Part I: Extracellular Molecules
Rodney B. Luwor* Department of Surgery, The Royal Melbourne Hospital, The University of Melbourne, Parkville, Victoria 3050, Australia
Abstract
Since its discovery several decades ago, the Epidermal Growth Factor Receptor (EGFR) has become one of the most extensively studies receptor tyrosine kinases. However, despite continued insight into the cancer promoting properties of the EGFR and its downstream signalling substrates, clinical use of agents targeting the EGFR continue to yield modest outcomes. Clinically, approved anti-EGFR therapeutics can successfully inhibit receptor activation. However major tumour regression is observed in only 10-30% of advanced unselected cancer patients, with most patients showing no therapeutic benefit. Furthermore, those who initially respond commonly relapse presenting with reoccurrence of tumours that are frequently resistant to the original therapy. In addition, the standard course of treatment of such agents is estimated to cost between “US $15,000-80,000/patient” for an improved overall survival of only 1-2 months. Therefore, it is both medically and financially critical to determine the true molecular mechanisms of tumour resistance, and how it can be overcome. In these 2 back-to-back chapters, we will provide an overview of the progress made in targeting the EGFR and discuss the challenges presented by the numerous molecular mechanisms currently identified, leading to overall refractory outcomes to anti-EGFR therapeutics. In this chapter (Part I) we will specifically focus on the resistance mechanisms driven by alterations in ligand and receptors of the EGFR family and cross-talk between EGFR receptors and non-EGFR family members.
Keywords: Afatinib, Cancer, Cetuximab, Epidermal Growth Factor Receptor, Erlotinib, Gefitinib, Lapatinib, Panitumumab, Resistance, Signaling, Therapeutics, Tumor.
* Corresponding author Rodney B. Luwor:Department of Surgery, The Royal Melbourne Hospital, The University of Melbourne, Parkville, Victoria 3050, Australia; Tel: +613 8344 3027 Fax: +613 9347 6488; E-mail: [email protected] 1. INTRODUCTION
Since the discovery of the Epidermal Growth Factor (EGF) in 1962 by Stanley Cohen and colleagues [1] tremendous advances in our understanding of the sophisticated interactions between growth factors and their accompanying cell surface receptors have been made. One of the most intensely studied classes of receptors is the HER or ErbB family [2]. This family consists of four members, the Epidermal Growth Factor Receptor (EGFR) (also referred to as ErbB1 or HER1) [3], HER2 (p185Neu or ErbB2) [4], HER3 (ErbB3) [5] and HER4 (ErbB4) [6]. All 4 family members share a similar overall structure consisting of an extracellular domain with 2 cysteine-rich regions, a single membrane-spanning region and a cytoplasmic domain containing multiple tyrosine residues that are phosphorylated upon receptor activation [7, 8].
The EGFR gene is located on the short arm of chromosome 7 [9, 10], and encodes an 1186 amino acid long, 140 KDa polypeptide chain [3, 11], which contains approximately 30 – 40 KDa of N-linked oligosaccharides [12, 13]. A single 23 amino acid long hydrophobic sequence transverses the cell membrane. The extracellular N-terminal end (amino acids 1 - 621) can be divided into four domains (I-IV) [14, 15]. The intracellular C-terminal region (amino acids 645 - 1186) is responsible for tyrosine kinase activity and regulatory functions [16].
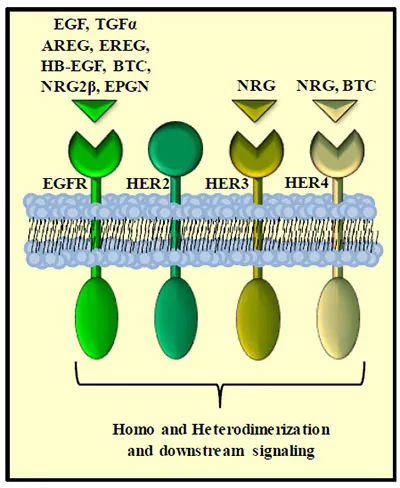
Currently eight ligands have been identified to bind the EGFR with varying affinity and potentially differential downstream function. They include EGF [1], transforming growth factor alpha (TGF() [17], amphiregulin (AR) [18], heparin-binding EGF-like growth factor (HB-EGF) [19], betacellulin [20], epiregulin [21], neuregulin-2-beta (NRG2β) [22] and the most recently discovered Epigen [23]. These peptide ligands are produced as trans-membrane precursors that are then processed by metalloproteases and released in their soluble form [24] (Fig. 1).
Ligand induced ATP binding to the EGFR lysine-721 residue is a critical step in tyrosine kinase activation and auto-phosphorylation in the intracellular region of the receptor [11, 25-28]. In turn, this auto-phosphorylation results in a more open conformation allowing access to several cellular substrates to the tyrosine kinase domain of the EGFR [25, 29] and subsequent triggering of downstream signaling cascades including the RAS-RAF-MAPK-Erk1/2 pathway, the PTEN regulated phosphatidylinositol 3-kinase (PI3-K)-Akt-mTOR pathway, Src-Signal transducer and activator of transcription (STAT) family members and the Phospholipase C gamma (PLCγ) signaling pathway [30]. These signaling networks and the evidence for alterations or hyper-activity of each of these downstream molecules in providing resistance mechanisms to anti-EGFR therapy will be covered thoroughly in Part II of our series of reviews.
Due to the EGFR’s many associations at the cell membrane and the diverse network of signaling, its activation is intimately associated with many cellular activities in both development and in the adult organism including proliferation, survival, differentiation, adhesion, migration and invasion and tumor metastasis. The importance of the EGFR in development is provided from the analysis of genetically altered mice. EGFR knockout mice display impaired epithelial development resulting in either embryonic or perinatal lethality or in mice suffered from abnormalities in multiple organs including the brain, skin, lung and gastrointestinal tract, depending on the genetic background [31-34]. Among the functions attributed to the EGFR are the proliferation and development of specific epithelial regions in the embryo, including branch point morphogenesis, maturation of early embryonic lung tissue, skin development and promoting survival of early progenitor cells in the cleft palate [35, 36]. The EGFR is also expressed throughout the brain during development primarily in the early postnatal astrocytes and purkinje cells [37, 38]. The EGFR also plays an important role in the adult organism where it is essential for the differentiation of normal mammary glands and the induction of uterine and vaginal growth [39, 40]. It is also required in the adult neurones of the cerebral cortex where it acts to promote terminal differentiation [41].
In summary these data clearly show the essential role of the EGFR during normal development and homeostasis. Not surprisingly, genetic alterations leading to EGFR over-expression or gain-of-function mutation are frequently observed in cancer [42-44]. These findings led to the vigorous pursuit that continues today to develop agents targeting the EGFR (and downstream substrates) in the hope that inhibition of EGFR-driven signal transduction will lead to improved cancer patient outcomes [45, 46]. However, despite the enormous effort and cost, only a very small percentage of tested agents have made it through clinical evaluation to be ultimately approved.
In this review we will particularly highlight the current inhibitors to the EGFR both in clinical application and being examined in translational models. We will also specifically focus on how ligands and receptors of the HER family and alternative non-EGFR family ligand-receptor pairs assist in by-pass therapeutic intervention from anti-EGFR agents and discuss potential strategies to overcome this resistance.
Fig. (1)) Schematic of HER family of ligands and receptors. The HER family r...