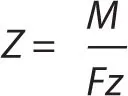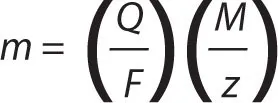![]()
1 History and Basics
Electroplating has been developed from an art form to an almost exact science, all driven by the developing needs of industries. Aerospace and microelectronic applications of electroplating used frequently today are beyond the comprehension of the first experimenters of electrodeposition. The first experiment involving the electrodeposition of metal is a little uncertain. It is widely accepted that the first experiment was performed in 1772 by an Italian professor of experimental physics, Giovanni Battista Beccaria [1]. His experiment used the charge from a Leyden jar, an early capacitor consisting of a glass jar and layers of metal foil, to decompose metal salts and deposit metal.
The development of electroplating has closely shadowed the development of electricity production with the first published experiment in gold plating occurring five years after the creation of Alessandro Volta’s, Voltaic pile. This early battery was used in the gold plating experiment by a colleague of Volta, Luigi Valentino Brugnatelli, in which the charge from the pile was used to facilitate the electrodeposition of a thin layer of gold from a solution onto two silver medals. Brugnatelli described how he had
No substantial progress was made to the development of electroplating due to the inadequacy of the Voltaic pile and other battery systems. There were, however, numerous scientists that were intrigued by electrochemical reactions (including electroplating), one of them being Michael Faraday. Among his other important discoveries and theories were his laws of electrolysis. After a few major experiments and the invention of the volta-electrometer, Faraday’s results had implied two laws:
1 The chemical deposition due to flow of current through an electrolyte is directly proportional to the quantity of electricity passed through it.
2 When the same quantity of electricity is passed through several electrolytes, the mass of the substances deposited are proportional to their respective chemical equivalent or equivalent weight.
The first law can be represented by the equation m ∝ Q, where m and Q are the chemical deposition and electric charge. It can also be written as m = ZQ, where Z is a constant of proportionality known as the electrochemical equivalent of a substance. The electrochemical equivalent can be expressed as
where M is the molar mass of the substance, F is the Faraday constant and z is the valency number of the substance. Faraday’s first law can therefore be expressed as:
This equation will be important later where it is used to work out the thickness or time of deposited material. These laws, along with a series of experiments, had given a sound qualitative and quantitative foundation for electroplating.
Invented in 1836 by British chemist, John Frederic Daniell, the Daniell cell [3] (a development of the Voltaic pile that allowed for a sustained, constant electrical output) provided electroplating with a suitable source of current for further experimentation, allowing for a more uniform coating of metal. Many scientists quickly took to experimenting with the new cell, one of whom was George Richards Elkington, who filed one of many Elkington patents on ‘An Improved Method of Gilding Copper, Brass and Other Metals or Alloys of Metals’ [4][5]. George, along with his cousin Henry, later filed many patents on electroplating. They were first interested in it as a technique to replace the hazardous gilding process, substituting these chemicals with materials that were less poisonous and easier to handle.
Later in their career, the Elkington cousins formed a partnership with John Wright, who discovered that gold and silver could be dissolved in potassium cyanide for use as an electrolyte in electroplating. A patent was hastily written and granted on the use of a cyanide electrolyte for gold and silver. The Elkingtons were granted further patents and eventually had great commercial success electroplating expensive-looking silverware and precious metals for very low cost.
Scientists in Russia, namely Moritz Hermann von Jacobi and Maximilian Herzog von Leuchtenberg, also took great interest in the Daniell cell. Initially, Jacobi repeated Daniell’s experiments, finding similar copper deposits mentioned in the first experiment. He then replaced the cathode with an engraved printing plate. The engraving was removed and Jacobi was left with a metal object that had a clear impression of the plate. This is now known as an electroform. Jacobi continued his work and reported his findings to the Academy of Sciences in St Petersburg, describing it as ‘galvanoplastik’, translated as electroforming. Jacobi was an advisor to Leuchtenberg who went on to create the St Petersburg Electroforming Company, one of the largest at the time with more than 800 employees. The company started off by producing electroforms for printing papers, but later went on to reproduce artworks by taking electroforms of statues and sculptures.
As the Industrial Revolution expanded from Great Britain to the rest of the world, the knowledge of electroplating began to broaden. Other types of plating processes, driven by specific manufacturing and engineering applications, were adapted for commercial usage. These included bright nickel plating, brass plating, tin plating and zinc plating.
After growth of the electroplating process in the Industrial Revolution, many new material advances were made. The scientific and mathematical models were developed to help to explain how the process worked and a few improvements were made in direct current power supplies, manufacturing methods and electrolyte solutions. The cyanide gold and silver solutions changed very little until, due to a surge in the electronics industry, the hazardous cyanide baths were mostly replaced with safer acid baths[6].
Today, the growing applications for the electronics, car and aerospace industry are beginning to push the boundaries of the structure of electrodeposited metal. There have also been, justifiably, further safety regulations, laws on emissions and disposal, chemical advancements and requirements for the entire electroplating industry. Many of the chemicals that have been used for generations are now being phased out in preference for safer and less detrimental alternatives to protect workers and the environment.
Faraday’s Nomenclature
It is interesting to know that Faraday, in correspondence with William Whewell [2], established the nomenclature associated with electroplating including the terms anodes, cathode, anion, cation, electrolysis and electrolyte.
WHAT MAKES A PLATING TANK?
The components of a plating tank, or in more general terms an electrolysis cell, consist of three main parts: the electrolyte, electrodes and electric circuit.
Electrolyte
The electrolyte is almost always aqueous where water is the solvent. Creation of an electrolyte solution would start with distilled, deionized (DI) or reverse osmosis (RO) water.
These types of water are similar in that the processes remove ionic impurities, but they are not interchangeable. Distilled water is demineralized water that has been purified through distillation. DI water is created by running water through an electrically charged resin. The ions in the resin react with ions in the water to produce more water. DI water is quite sensitive and will start to react with air as soon as it comes into contact. Carbon dioxide from the air will dissolve and react with molecules into the water, forming hydrogen ions and bicarbonate. It also does not remove any impurities in the water, only charged particles. Diffusion is the movement of molecules from an area of high concentration to an area of low concentration until a thermodynamically favourable equilibrium is reached. Osmosis is a special case of diffusion where the molecules are water and the concentration gradient is a semipermeable membrane. The best type of water for an electrolyte would be RO water.
Ionic crystals are the next component in the creation of an electrolyte solution. These usually take the form of a metal salt lattice; MA is an example we will use here, where M is the metal and A is the salt. The salt can also be written as a product of two ions Mz+ Az-...