“Fit for Purpose” 64Cu Labeled Liposome Formulations Specialized for Enriched Targeting to 1) Bone Marrow Spleen; 2) lymph Nodes; or 3) Tumor
Sang-Gyu Lee1, *, Kishore Pillarsetty1, Steven M. Larson1 1 Larson Lab, Molecular Pharmacology Program, Sloan Kettering Institutes, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Abstract
Upon intravenous injection, liposomal particles are rapidly cleared from the blood by fixed macrophages in the liver, spleen, bone marrow and lymph nodes i.e. specialized cells of the innate immune system. By reducing the affinity for these fixed macrophages, therapeutic liposomes containing drugs have been used to target tumor by the Enhanced Permeability and Retention (EPR) effect. In this communication, we report minor modifications of lipid compositions that result in the production of 3 classes of liposomes, each suitable for enhanced selectivity of uptake in 1) Bone Marrow/Spleen (SBMT Lipo); 2) Lymph Nodes (LNT Lipo); and 3) Tumor (TT Lipo).
Keywords: Bone Marrow Imaging, Copper-64, Endothelial Macrophages, Lymph Node Imaging, Nanoparticles, PET, Reticuloendothelial System, Tumor Imaging.
* Corresponding author Sang-Gyu Lee: Larson Lab, Molecular Pharmacology Program, Sloan Kettering Institutes, Memorial Sloan Kettering Cancer Center, New York, NY, USA; E-mail: [email protected] Introduction
For many years nuclear medicine has exploited the cells of the fixed macrophage system for imaging purposes by using microscopic particles as radiaotracers. Initially, Au-198 colloid and succeeded by Tc-99m colloid were used to imaging liver, for detection of metastatic tumors, as well as bone marrow to identify sites for biopsy. More recently, a variety of simple radiolabeled particles are used for lymphatic imaging upon subcutaneous injection, i.e. sentinel lymph node imaging for breast cancer and melanoma staging. These have also included a variety of nanoparticles of various materials and sizes.
In the current manuscript, by modulating the size and surface lipid composition,
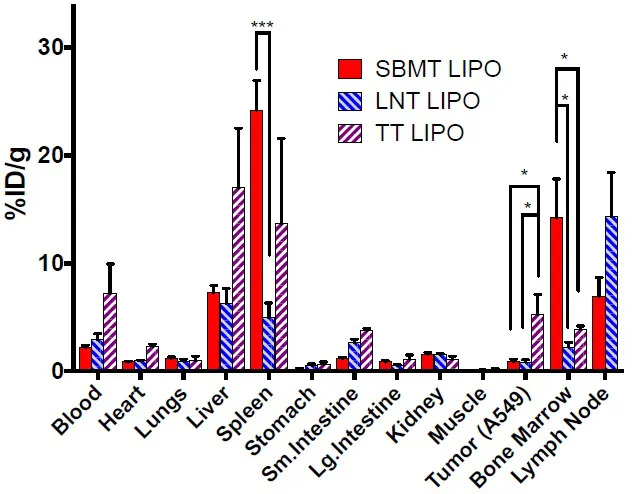
we have developed liposomal formulations that allow for enhanced targeting to organ-specific cells of the fixed macrophage system (aka Reticuloendothelial System (RES), after parenteral administration. Liposomes in the size range of ~90 nanometers in diameter with varying degrees of pegylation and surface charge were developed for specific targeting purposes. For example, SBMT Lipo preferentially accumulates in the phagocytic cells lining the bone marrow and spleen sinusoids [1]. SBMT Lipo formulation was used to provide enhanced delivery of radioprotector drug γ-tocotrienol (GT3), or GT3-Nano for short, to the spleen and bone marrow, to mitigate bone marrow radiation damage from external beam radiation or targeted radionuclide therapy (TRT). Fig. (1) shows the biodistribution profile of different “fit for purpose” liposomal formulations that can be used for distinct purposes, including imaging, drug delivery.
Fig. (1)) Biodistribution of 64Cu labeled selective targeting liposomes at 24 h post-injection. Athymic nude mice bearing A549 tumor were injected with ~140 μCi (5.18 MBq) of
64Cu labeled liposomes. 1.4 mg (2 μmoles) of lipid was injected each mouse through tail vein injection and it is corresponding to approximately 6 x 10
12 liposome particles per mouse. Mice were euthanized at 24 h post-injection and organs were harvested in pre-weighed tubes to measure organ weight and γ-counting. Data were presented as mean and SEM. n=5 per group. * p < 0.05, *** p < 0.001.
Methods
The compositions of each liposome are as follows; SBMT Lipo. Phosphatidylcholine (PC), cholesterol, succinyl phosphoethanolamine (SuPE) and mPEG2k-DSPE (6:3:1:0.1). LNT Lipo. The ratio was adjusted to 6:3:1:0.7. TT Lipo, the ratio between PC, cholesterol, and mPEG2k-DSPE are 7:3:0.5. Tumor targeting no SPE MPEG 5%. All liposomes were manufactured by the standard extrusion method at 65 oC and the sizes of liposomes are 90 nm. The formulation was stable for 2 years at 4 oC in size and 64Cu labeling to the liposomes. Labeling was performed with 64Cu chelation to DOTA-bn- DSPE doped liposomes after incubation of [64Cu] CuCl2 at 37 C for 1 hour. ITLC was used for quality control, and yield was quantitative [2].
Animal studies. Mice were injected intravenously by tail vein and ex-vivo biodistribution was performed at 24 hours post-injection. Mice were imaged 24 h post liposome administration using Focus 120 microPET or Inveon PET/CT scanner.
Results and Discussion
Using 3 different formulations, mice were sacrificed, and biodistribution was performed at 24 hours after injection of approximately 6x1012 particles (60 pmoles), of liposomal preparations of approximated 90 nm in diameter of 3 stable formulation types: SBMT Lipo, LNT Lipo, and TT Lipo. 64Cu, a positron emitter, provides a convenient radiotracer for gamma counting and/or PET imaging. (1) A comparison of the biodistribution results from independent studies is shown in Fig. (1). All 3 formulations target the spleen, bone marrow, liver and lymph nodes. However, by altering the surface charge and/or pegylation fraction, major changes in relative biodistribution occur. As is clear from Fig. (1), there are statistically significant distribution differences between the tissues, and this translates into major differences in PET imaging appearance. In Fig. (1), in one case, a tumor was targeted and comparison, in this case, is between the SBMT Lipo and the TT Lipo. Tumor uptake is most favorable in this TT Lipo formulation, with a mean change greater than 5 times the uptake in tumors seen with the SBMT LIPO.
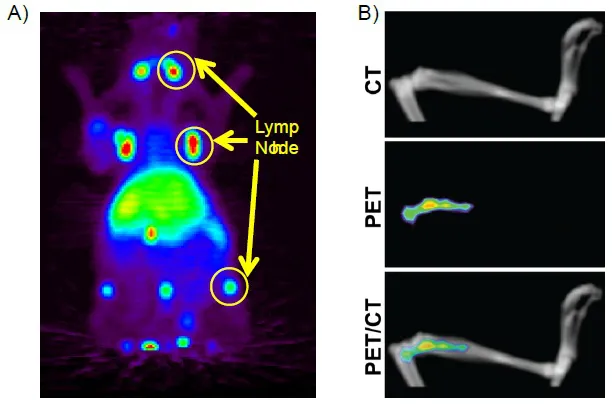
The LNT Lipo steers liposomes to the lymph node, as shown in Fig. (2A). The SBMT Lipo formulation steers the liposome to the spleen and bone marrow at the expense of the liver uptake Fig. (2B). The preparation was developed to deliver cargo as selectively as possible to the spleen and bone marrow, wherein stem cells reside that are important for the regeneration of bone marrow, damaged by radiation effects. A recent publication in the JNMMI [2] describes a newly developed radioprotector drug liposomal formulation GT3-Nano based on SBM Lipo formulation that carriers radioprotector Gamma-tocotrienol (GT3), a form of Vitamin E,. This formulation promotes recovery of hematopoietic function post-radiation. Among the uses will be protected against radiation toxicity from TRT approaches under active development in multiple clinical trials. This protection may afford higher dose delivery to achieve greater efficacy of TRT in man While minimizing toxic effects on bone marrow.
Fig. (2)) MIP images of mice labeled with 64Cu to
A) lymph node targeting liposomes.
64Cu was chelated to DOTA-Bn-DSPE containing liposomes. 100 uCi was administered by tail veil injection and the image was taken at 24 hours post-injection. and
B) volume-rendered images showing CT, PET/CT fusion and PET images of tibia demonstrate that accumulation of liposomes is specific to bone marrow.
In summary, minor modifications of manufacture can alter the biodistribution of liposomal particles within the fixed macrophages of the innate immune system (RES). They can benefit from the delivery of drugs for specific purposes to a pre-selected region of the body. Further studies are underway to explore the utility of this approach, which is image-guided by radionuclides useful for biodistribution and quantitative PET imaging.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The author confirms that this chapter contents have no conflict of interest.
ACKNOWLEDGEMENTS
This study was supported in part by the MSKCC Center for Molecular Imaging and Nanotechnology grant and the Cancer grant from the National Cancer Institute (P50-CA086438). Technical services provided by the MSKCC Small-Animal Imaging Core Facility, supported in part by NIH Cancer Center Support Grant No. 2 (P30-CA008748-48), are gratefully acknowledged. NIH Shared Instrumentation Grants (S10-RR020892-01 and S10-OD016207-01) provided funding for the purchase of the Focus 1...