![]()
Tucker AS, Miletich I (eds): Salivary Glands. Development, Adaptations and Disease.
Front Oral Biol. Basel, Karger, 2010, vol 14, pp 48–77
______________________
Extracellular Matrix and Growth Factors in Salivary Gland Development
Sharon J. Sequeira · Melinda Larsen · Tiffany DeVine
Department of Biological Sciences, University at Albany, SUNY, Albany, N.Y., USA
______________________
Abstract
The interstitial extracellular matrix (ECM) and epithelial-cell associated basement membrane (BM) play critical roles in the morphogenesis and differentiation of developing salivary glands. Early studies used ex vivo organ culture and tissue recombination methods to identify the importance of the ECM in organ development. Incorporation of transgenic mice and molecular tools has facilitated progress in our understanding of the mechanisms by which ECM proteins influence SMG development. Recent work has identified alterations in the ECM, BM, and associated proteins in salivary gland diseases, including Sjögren‘s syndrome and salivary gland cancers, but the significance of such changes is not known. Understanding the basic mechanisms controlling morphogenesis and differentiation in mammalian organ development is the first step towards understanding pathogenesis. Molecular characterization of the function of the ECM and BM in cellular processes is critical for future development of therapeutic approaches in regenerative medicine and tissue engineering .Here we provide a historical overview of experiments defining the functions of the ECM, ECM receptors, and associated molecules in salivary gland development. We include a discussion of the function of ECM-associated proteases and major growth factor signaling components that are in some way regulated by the ECM or associated molecules. We conclude with a discussion of defects in ECM and BM occurring in salivary gland pathologies and speculation on future areas of research pertaining to further understanding of the function of the ECM in the salivary gland.
Copyright © 2010 S. Karger AG, Basel
The submandibular, or submaxillary, salivary gland (SMG) initiates from a thickening in the oral epithelium that grows into the neighboring mesenchymal, or stromal, tissue. Once this epithelium develops into a primary bud attached to an elongated stalk, the process of branching morphogenesis begins, which ultimately results in the complex arborized structure that is the adult gland [reviewed in 1, 2].The SMG has long been studied as a model system for the study of branching morphogenesis since the developing SMG can be removed from the embryo as early as embryonic day 12 (E12), with the day of plug discovery defined as day 0, and studied ex vivo as an organ culture [3, 4]. The process of cellular cytodifferentiation, whereby the cells take on their ultimate shape and function, occurs during the later stages of morphogenesis through independent mechanisms that also require the influence of extracellular matrix (ECM) proteins [5]. Studies performed over the past 60 years have provided insight into the mechanisms whereby the stromal-derived ECM and the specialized epithelial cell-associated ECM, the basement membrane (BM), influence the growth, morphogenesis, and differentiation of the SMG tissues.
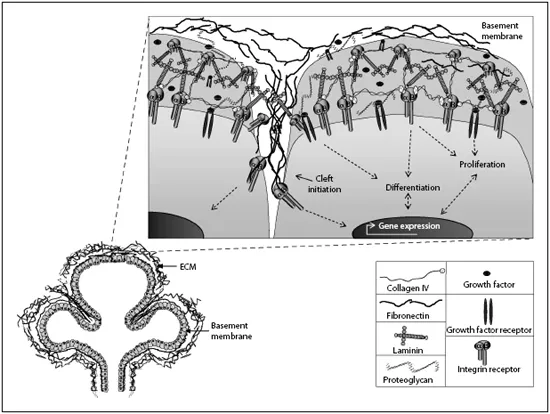
The ECM is a network of macromolecules located within the stromal connective tissue compartment of organs that is constructed by the stromal cells. In the SMG, the ECM is a significant component of the developing organ, but it accounts for a diminishing volume of the tissue in the adult gland. The ECM is composed of networks of fibrillar proteins, network accessory proteins, hydrophilic heteropolysaccharides (glycosaminoglycans) that are either not attached (e.g. hyaluronan) or are attached to proteins (proteoglycans), growth regulatory proteins that are sequestered in the network, and proteases and their inhibitors that regulate cleavage of ECM and associated proteins. The structure of the ECM gives it strength and rigidity, in addition to multiple other functions. The ECM is tightly linked to cell membranes through direct attachment via cell-surface receptor proteins (i.e. integrins, and other transmembrane proteins) and, through these signal-transducing transmembrane receptors, directs cellular responses, including cell differentiation, migration, and polarization. Signals initiated from inside the cell cytoplasm are also transmitted through these transmem-brane receptor proteins to the ECM, resulting in its modification and its dynamic remodeling [6, 7].
The BM is a specialized form of ECM, located directly adjacent to the epithelium that is primarily synthesized by these cells and influences both morphogenesis and cell differentiation. BMs are generally composed of two thin sheets of matrix proteins: a layer known as the lamina densa (30-70 nm) and an underlying layer of reticular fibers (3 nm), which are collectively known as the BM [8, 9]. Scanning electron microscopic studies of developing rat SMG suggested that the two layers are significantly thicker than this: 100-400 and 30-40 nm, respectively at the E16 stage [10].Early studies documented the importance of the BM in maintaining the structure of the epithelial lobules and implied that the BM participates in regulating the process of branching morphogenesis [11-15] and in facilitating exocrine secretion by the acinar structures [16]. The BM is a dynamic structure that undergoes remodeling during salivary gland morphogenesis and differentiation [17-19] and participates in mediating changes in tissue shape during morphogenesis [20].
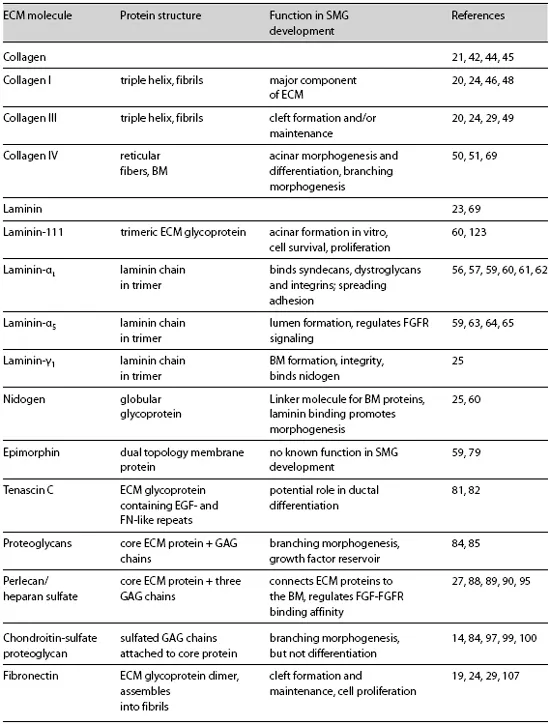
Many ECM molecules are known to play an active role in salivary gland morphogenesis and differentiation, and their specific functions will be discussed here. These functions are summarized in figure 1. Collagens [21] are an important component of the ECM. The BM includes laminins [22, 23], collagen type IV [24], and nidogen[25, 26]. Glycosaminoglycans [11], proteoglycans [12], and heparan sulfate proteoglycans, such as perlecan [27, 28], are also integral components of the BM (table 1). Fibronectin is a component of both the stromal ECM and the BM that is produced both by the mesenchyme [22] and by the epithelium and is critical to morphogenesis [29]. The ECM also serves as a storehouse for growth factors and as a mediator of growth factor interactions with their receptors. Proteases and protease inhibitors are involved in the release of growth factors and growth regulatory fragments of ECM proteins. Here we focus on studies that have impacted our understanding of the diverse functions that the ECM, BM, and associated molecules perform during SMG development. Although the other salivary glands (parotid, sublingual, and minor salivary glands) develop similarly, this discussion will focus primarily on the SMG. The majority of the studies mentioned were performed using mouse or rat cells and tissue, but most of the mechanisms discussed are likely to be conserved during human development.
Fig. 1. Schematic of ECM-cell interactions during salivary gland development.
Table 1. Summary of ECM and BM proteins involved in submandibular gland development
Three-Dimensional Cell and Tissue Models
Our understanding of the function of ECM proteins in the developmental process of branching morphogenesis has greatly benefitted from ex vivo organ culture. In this technique, intact organs and tissues are harvested from live embryonic or adult mouse tissue and cultured in vitro for a finite period of time, during which the initial three-dimensional (3D) structure is maintained (fig. 2) [3, 4]. Borghese [30] was the first to realize that the mesenchymal tissue compartment was critical for the development of the epithelial tissue. Studies using tissue recombination models, in which epithelium from one tissue source is combined with the mesenchyme from another and then grown in a permissive in vitro environment, have demonstrated the inductive influence of the surrounding mesenchymal tissue and its accompanying ECM on the epithelial phenotype. The instructive function of SMG mesenchyme is clear from experiments in which it was combined with pituitary epithelium. The resulting epithelium within this tissue hybrid not only shows epithelial morphology, but also produces the salivary product, α-amylase, indicative of acinar differentiation [31]. The tissue recombination technique as performed here did not elucidate which specific molecules were involved. However, in many experiments, a Transwell filter placed between the epithelial and mesenchymal tissue did not negate the instructive function of the mesenchyme [32], indicating that the functional molecule(s) may be a soluble factor or a factor that can physically connect with the tissue through the pores of the filter. Studies later predicted that a soluble factor was necessary since the signals could be transmitted through a pore size as small as 0.05 μm [33].
In the embryonic salivary gland model, SMG epithelial cells dissociated from the surrounding mesenchyme will not grow alone. However, they can continue to undergo development if co-cultured with mesenchyme cells, implanted within an ECM gel and co-cultured with mesenchyme cells [33], or implanted in an ECM gel supplemented with mesenchymal-derived growth factors, such as fibroblast growth factors (FGFs) or epidermal growth factor (EGF) [34, 35]. The first ECM extract to be produced, which is still the most frequently used extract for 3D culture, was a BM extract derived from the Engelbreth-Holm-Swarm tumor tha...