1.1 Introduction
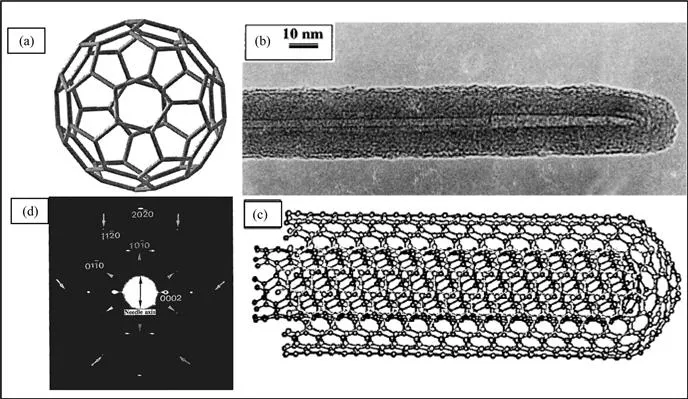
Diamond and graphite are the two well-known forms of crystalline carbon. Diamond has four-coordinate sp3 carbon atoms that form an extended three-dimensional network, whose motif is the chair conformation of cyclohexane. Graphite has three-coordinate sp2 carbons that form planar sheets, whose motif is the flat six-membered benzene ring. The new carbon allotropes, the fullerenes, are closed-cage carbon molecules with three-coordinate carbon atoms tiling the spherical or nearly-spherical surfaces, the best known example being C60, with a truncated icosahedral structure formed by 12 pentagonal rings and 20 hexagonal rings (Figure 1.1a). Fullerenes were discovered by Kroto et al.1 in 1985 while investigating the nature of carbon present in interstellar space. The coordination at every carbon atom in fullerenes is not planar, but slightly pyramidalized, with some sp3 character present in the essentially sp2 carbons. The key feature is the presence of five-membered rings, which provide the curvature necessary for forming a closed-cage structure. In 1990, Krätschmer et al.2 found that the soot produced by arcing graphite electrodes contained C60 and other fullerenes. The ability to generate fullerenes in gram quantities in the laboratory, using a relatively simple apparatus, gave rise to intense research activity on these molecules and caused a renaissance in the study of carbon. Iijima3 observed, in 1991, that nanotubules of graphite were deposited on the negative electrode during the direct current arcing of graphite for the preparation of fullerenes. These nanotubes are concentric graphitic cylinders closed at either end due to the presence of five-membered rings. Nanotubes can be multi-walled with a central tubule of nanometric diameter surrounded by graphitic layers separated by ∼3.4Å. Unlike in multi-walled nanotubes (MWNTs), in single-walled nanotubes (SWNTs), there is only the tubule and no graphitic layers. A transmission electron microscope (TEM) image of a MWNT is shown in Figure 1.1b. In this nanotube, graphite layers surround the central tubule. Figure 1.1c shows the structure of a nanotube formed by two concentric graphitic cylinders, obtained by force-field calculations. A single-walled nanotube can be visualized by cutting C60 along the centre and spacing apart the hemispherical corannulene end-caps by a cylinder of graphite of the same diameter. Carbon nanotubes are the only form of carbon with extended bonding and yet with no dangling bonds. Since carbon nanotubes are derived from fullerenes, they are referred to as tubular fullerenes or bucky tubes.
Figure 1.1 (a) Schematic diagram of a C60 molecule. (b) A TEM image of a multi-walled carbon nanotube. (c) Minimum energy structure of a double-walled carbon nanotube. (Reproduced from ref. 31a). (d) Electron diffraction pattern of a multi-walled carbon nanotube. (Reproduced from ref. 31c).
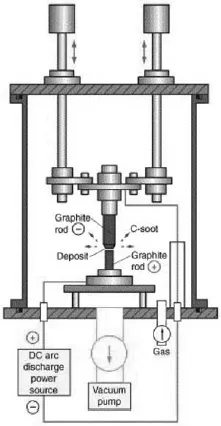
Ever since the discovery of carbon nanotubes,3 several ways of preparing them have been explored.4,5 Multi-walled (MWNTs), double-walled (DWNTs) and single-walled (SWNTs) carbon nanotubes have been synthesised using methods which include arc evaporation of graphite, laser ablation, chemical vapour deposition (CVD) and vapour phase decomposition or disproportionation of carbon-containing molecules.6,7 Kumar and Ando8 have reviewed the synthesis, growth mechanism and mass production of carbon nanotubes by CVD. MWNTs and SWNTs have also been prepared by techniques such as electrochemical synthesis9 and pyrolysis of precursor organic molecules.10 Among the various types of carbon nanotubes, SWNTs are of special interest because of their unique properties and potential applications. Specific methods have been found to obtain long SWNTs11 as well as diameter-control of the nanotubes.12 SWNTs of various forms such as rings,13 brushes14a and films14b have been prepared. Vertically aligned15 as well as horizontally aligned16 carbon nanotubes have been prepared on different substrates by several workers. Various methods as well as new strategies employed for the enrichment or selective generation of SWNTs with metallic, semiconducting and other unique electronic properties has been reported.17 Diameter selective dispersions of SWNTs are reported.18
The structure of carbon nanotubes has been extensively investigated by high-resolution electron microscopy.19–21 Nanotubes, prepared by arc vaporization of graphite, are closed at both ends, but can be opened by various oxidants.22,23 There has been considerable success in filling nanotubes with various materials.24 Apart from opening and filling, carbon nanotubes have been doped with boron and nitrogen, giving rise to p-type and n-type materials respectively. By employing carbon nanotubes as removable templates, oxidic, carbidic and other nanostructures have been prepared. Aligned nanotube bundles have been grown for specific applications. Properties and phenomena as well as several possible and likely applications of carbon nanotubes have been reported extensively. Unsurprisingly, therefore, these nanomaterials have elicited great interest. Several review articles, special issues of journals and conference proceedings25–31 have dealt with carbon nanotubes. Some of the reviews present possible technological applications, with focus on the electronic properties,30,31 the book of Reich et al.32 being devoted to a detailed presentation of the basic physics of carbon nanotubes. There are several other reviews and books as well, some of which are cited as references.33–38
Since the discovery of the carbon nanotubes, there has been considerable work on inorganic layered materials such as MoS2, WS2 and BN to explore the formation of nanotubes of these materials. Indeed several have been synthesized and characterized.39–42 Inorganic nanotubes are discussed at length in Chapter 2. Here, we shall present various aspects of carbon nanotubes, which include their preparation, structure, mechanism of formation, chemical modification, functionalization, properties and applications. Specifically, we discuss their electronic structure and related properties, vibrational, thermal characteristics and mechanical properties. These aspects are interrelated, since both thermal and mechanical properties reflect the chemical bonding in the carbon network, which controls their electronic structure as well.