G. VERHOEVENa, b
a Ludwig Boltzmann Gesellschaft GmbH, LBI for Archaeological Prospection and Virtual Archaeology, Franz-Klein-Gasse 1, Wien, 1190, Austria;
b Ghent University, Department of Archaeology, Sint-Pietersnieuwstraat 35 (110-016), Gent, 9000, Belgium
E-mail:
[email protected] 1.1 Introduction
In the fields of cultural heritage, archaeology, art history and museology, many examination methods are based on imaging techniques. To date, numerous different imaging approaches exist, and although most of them have already existed for several decades, recent advantages in hard- and software technology make their application more straightforward than ever. In this chapter, an elementary but essential coverage of photodetectors, illumination equipment and signal composition will be provided to better understand the basic principles of common non-destructive scientific visualisations such as broadband colour photography, non-visible ultraviolet and infrared imaging, fluorescent photography, spectral imaging and X-ray radiography. Although important, many means to characterise and depict archaeological and heritage object will not be discussed (such as particle induced X-ray emission or PIXE, ultrasound techniques, thermography, stereomicroscopy, computed tomography, neutron imaging), nor will the file formats needed to store these visualisations and many other related archiving issues. For those interested in these topics, the following chapters of this book and the excellent compendium of MacDonald1 are recommended. Although several practical examples will illustrate each and every visualisation technique tackled in this chapter, it is still necessary to go back to some basic physics. Since imaging art objects and archaeological artefacts are all based upon some essential principles of how matter interacts with radiant energy, this chapter will start with a concise exploration of the world of matter, charge and energy.
1.1.1 Electromagnetic Radiation
Matter can be described as anything that occupies space and has weight. It is constituted by elements in various combinations. These elements, all described in the periodic table, are made up of atoms. Such atoms can be seen as the smallest matter particle, retaining all physical characteristics of an element. Different atom models were proposed during the last two centuries. Today, the Bohr model—introduced by Niels Bohr in 1913—is often used, as it offers an easy way to depict the atom besides a relative correctness. In this model, the central core or nucleus of the atom is made up of chargeless neutrons and positively charged protons, whose amount is indicated by the atomic number Z. To compensate for these positive charges, negatively charged particles or electrons orbit this nucleus. Hence, it is correct to say that electric charge is substance-like and that all physical objects are composed of electric charge.
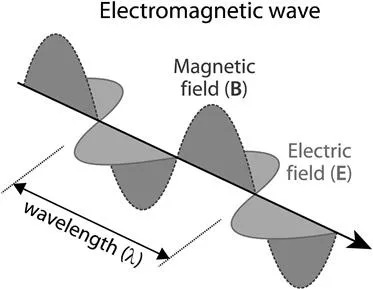
Charge differs from energy. Energy should not be looked at as a substance but rather as an attribute of a system that always turns out to be conserved. To be able to track energy flows, energy can be conceptualised by the model of fields. In the same way that a massive object can produce a gravity field to which distant objects respond, electrical charges and magnets alter the region of space around them so that they can exert forces on distant objects. It is exactly this altered space that is called a field (more technically, these fields are just vector quantities). Scientists have known since the early part of the 19th century that electrical fields and magnetic fields are intimately related to each other: moving electric charges (i.e. electric current) create a magnetic field and a changing magnetic field creates electrical current (thus electrical fields). A consequence of this is that changing electric and magnetic fields should trigger each other. The Scottish mathematician and physicist James Clerk Maxwell (1831–1879) put these ideas together and mathematically described the relationship between the magnetic and electric fields, as well as the currents and charges that create them. To conclude this line of reasoning, Maxwell said that visible light is an electromagnetic (EM) wave, consisting of both an electric (E) and magnetic (B) field (Figure 1.1). Both fields are oscillating perpendicular to each other as well as perpendicular to the direction of propagation (which makes them a transverse wave), whilst propagating at 299 792 458 m s−1 in vacuum. The latter speed is known as the speed of light, denoted c, and decreases when light travels in air, glass, water or other transparent substances.2–4
The electric and magnetic component vectors vibrate in phase and are sinusoidal in nature: they oscillate in a periodic fashion as they propagate through space and have peaks and troughs (see Figure 1.1). Being a self-propagating and periodic wave-like phenomenon, EM radiation is distinguished by the length of its waves, called the wavelength (λ), its magnitude of change or amplitude (A) as well as its frequency (ν): a figure—expressed in Hertz (Hz)—that indicates the number of complete waves or sinusoidal cycles passing a certain point in one second and thus inversely proportional to λ. No matter what portion of this broad spectrum is considered, they all obey the same physical laws and the relation c = λν holds for each.
Figure 1.1 An electromagnetic (EM) wave consisting of electric and magnetic oscillating fields. In this example, the oscillating magnetic field vectors are indicated with a dotted line.
1.1.2 Light and Beyond-visible Radiation
The principle that allows the human visual system to observe objects and persons is based on those subjects’ reflection of visible light. Dark objects do not reflect much incoming light, whereas a healthy banana principally reflects yellow light. However, this light is only one small portion out of the complete, so-called EM spectrum radiated by the sun or other sources (like stars or lamps). The EM spectrum can be considered a continuum of varying EM waves, all consisting of electric and magnetic fields that are described by the Maxwellian theory, but distinguishable by their wavelength.
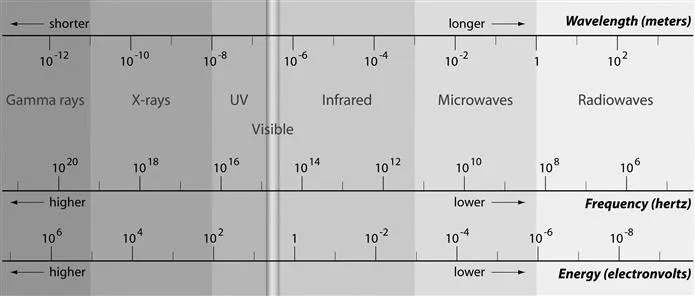
Visible light (“light”), for instance, is only a very narrow spectral band with wavelengths ranging from approximately 400 nm (400 × 10−9 m) to 700 nm (700 × 10−9 m), the absolute thresholds varying from person to person and specific viewing conditions. These wavelengths correspond with frequencies comprised between 7.49 × 1014 Hz (749 THz) to 4.28 × 1014 Hz (428 THz). Nonetheless, the complete EM spectrum consists of far more particular wavebands with characteristic frequencies and related wavelengths that are not perceivable by the unaided normal human eye. To both sides of the visible band there is EM radiation which does not produce a visual sensation: gamma rays, X-rays, and ultraviolet (UV) rays with shorter-than-visible wavelengths (and higher frequencies), while infrared (IR) rays, microwaves, and radiowaves can be found in the long-wavelength, low-frequency region (Figure 1.2).
Figure 1.2 The EM spectrum.
In addition to the aforementioned wave properties, EM radiant energy is known to exhibit particle-like behaviour and can be seen as a travelling bundle of photons (i.e. discrete energy packets) with energy levels that differ according to the wavelength. EM radiation can thus be considered a vehicle for transporting energy from the radiation source to a destination, photons being the particles of EM energy. To calculate a photon’s quantum energy (E), P...