
Data Integrity and Data Governance
Practical Implementation in Regulated Laboratories
R D McDowall
- 598 Seiten
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
Data Integrity and Data Governance
Practical Implementation in Regulated Laboratories
R D McDowall
Über dieses Buch
Data integrity is the hottest topic in the pharmaceutical industry. Global regulatory agencies have issued guidance, after guidance after guidance in the past few years, most of which does not offer practical advice on how to implement policies, procedures and processes to ensure integrity. These guidances state what but not how. Additionally, key stages of analysis that impact data integrity are omitted entirely.
The aim of this book is to provide practical and detailed help on how to implement data integrity and data governance for regulated analytical laboratories working in or for the pharmaceutical industry. It provides clarification of the regulatory issues and trends, and gives practical methods for meeting regulatory requirements and guidance. Using a data integrity model as a basis, the principles of data integrity and data governance are expanded into practical steps for regulated laboratories to implement. The author uses case study examples to illustrate his points and provides instructions for applying the principles of data integrity and data governance to individual laboratory needs. This book is a useful reference for analytical chemists and scientists, management and senior management working in regulated laboratories requiring either an understanding about data integrity or help in implementing practical solutions. Consultants will also benefit from the practical guidance provided.
Häufig gestellte Fragen
Information
Data Integrity is the hottest topic in the pharmaceutical industry now and will continue to be in the future. Regulatory authorities around the world have issued a number of guidance documents on the data integrity and data governance since the start of 2015 as well as two industry guidance documents. However, all documents are vague and most do not contain detailed examples or advice to help regulated laboratories to implement policies, procedures and processes to ensure integrity: they outline what needs to be done but there is often insufficient detail for effective implementation. From an analytical perspective there has not been a detailed focus on data integrity in a regulated analytical laboratory. Hence, the rationale for writing this book.
1.1 Aims and Objectives
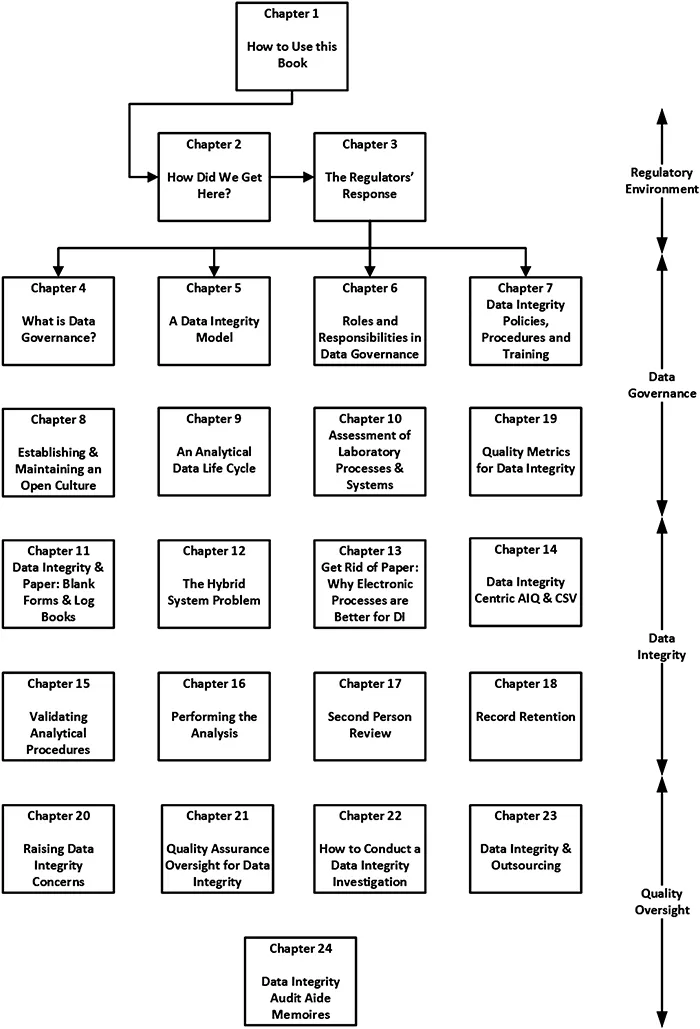
1.2 Structure of This Book
- How to Use this Book is covered in this chapter.
- The Regulatory Environment is discussed in Chapters 2 and 3.
- Data Governance is presented and explained in Chapters 4 to 10 as well as Chapter 19.
- Data Integrity is covered in Chapters 11 to 18.
- Quality Assurance Oversight and Outsourcing are discussed in Chapters 20–24.
1.2.1 Chapter Structure
- A chapter starts with a brief overview why the chapter is important within the overall context of data integrity and data governance.
- This is followed by a section on regulatory requirements or regulatory guidance that are relevant to the chapter; thereby positioning the regulatory rationale for the topic of the chapter.
- Where appropriate, there is also the business rationale for the tasks contained in the chapter.
- Then there is a discussion of how to achieve the objective of each chapter. For example, if you are assessing a process or a computerised system the chapter discusses how this can be achieved and how to avoid some of the common pitfalls.
- Each chapter finishes with a list of the references used.
1.2.2 You Do Not Read the Regulations!
1.2.3 The Regulatory Environment
- Chapter 2, entitled How Did We Get Here?, provides the background to data integrity in regulated laboratories of the pharmaceutical industry over the past quarter of a century. The story starts with the Barr Laboratories court case and ruling in 1993 1,3 and then moves on to the Able Laboratories fraud case in 2005.4 The latter case triggered regulatory authorities to change their inspection focus from paper to electronic records and consequently they discovered many more cases of data falsification and poor data management practices in companies throughout the world. The key compliance issues, derived mainly from FDA warning letters for paper records and computerised systems, are used to highlight areas of regulatory concern for laboratory operations.
- Chapter 3 is The Regulators' Responses and outlines the response to the increased occurrence of data falsification and poor data integrity practices by the various regulatory agencies by issuing guidance documents and where necessary updating regulations. This chapter looks at the various guidance documents issued. The first was the FDA's Inspection of Pharmaceutical Quality Control Laboratories issued in 1993 following the Barr Laboratories case.5 Since 2015 there have been a many data integrity guidance documents issued from regulatory agencies and industry bodies.6,12
1.2.4 Data Governance
- Chapter 4, entitled What is Data Governance?, sets out the strands of data governance that are discussed in more detail in the rest of the book. Data governance is not new and has been used outside the pharmaceutical industry for at least 15 years. We will compare and contrast DG definitions from inside and outside the industry. From the data governance definitions and input from the various regulatory and industry guidance documents we will draw out the key features of a programme of work: roles and responsibilities, DI policy and training, culture and ethics, open and honest approach to work as examples of a corporate DI policy. Are there areas in an organisation, e.g. research, where data integrity should not apply?
- Chapter 5 presents a Data Integrity Model13 consisting of four layers that describes data integrity within a pharmaceutical company from a GMP perspective: production, quality control and quality assurance, however, this book will focus on an analytical laboratory with quality assurance oversight. The four layers of the Data Integrity Model comprise:
- Foundation: data governance and setting right culture and ethos for data integrity.
- Level 1: Ensuring the right system and right instrument for the job.
- Level 2: Developing and validating the right analytical procedure for the job.
- Level 3: Allying all lower layers of the model to ensure the right approach for the right reportable result.
- Chapter 6 focuses on all those involved with data governance and outlines the key Roles and Responsibilities of a Data Governance Programme. Here, the various roles, from the boardroom to the laboratory bench, are presented and the responsibilities of each one discussed. The roles of process owner and system owner presented in EU GMP Annex 11 16 will be mapped to the data governance roles so that the roles are aligned with the regulations. We will also see how a corporate data integrity programme impacts a regulated laboratory.
- Chapter 7 discusses Data Integrity Policies, Procedures and Training. Data integrity policies and associated procedures must be present throughout an organisation. These will vary from an overall data integrity policy, good documentation practices for paper and computerised systems, interpretation of laboratory data and second person review. Coupled with the policies and procedures there must be effective staff training and where necessary evidence of the effectiveness of training. Typical poor documentation practices and record keeping failures are highlighted.
- Chapter 8 covers the necessity for Establishing and Maintaining an Open Culture for Data Integrity. It will explore what is open culture as well as the role of senior and laboratory management in setting expectations and maintaining a culture to ensure data integrity. This will include ways of balancing the pressure of work that could result in staff cutting corners due to management pressure that could compromise data integrity. This is the most difficult part of the Data Integrity Model as it requires management leadership and will require constant nurturing.
- Chapter 9 presents An Analytical Data Life Cycle from acquisition through processing, retention and finally destruction at the end of the retention period. The currently published data life cycles are inadequate as they do not cover analytical procedures in any detail as they are generic. Presented here is a flexible analytical data life cycle from sampling to reporting that can adapt to any analysis from simple to complex. There are potential problems as some analyses do not generate objective evidence that is essential to demonstrate that an activity took place and for second person review. Key phases of the life cycle are sampling, sample preparation, acquisition and interpretation and these are where poor practices and falsification can occur, often without detection. Reit...