![]()
1 What is a Photocatalyst?
1.1 Function and Utilization of Materials
Materials supporting our modern lives are composed of various combinations of numerous substances. The substances are mostly elements or chemical compounds, which are often in solid states. Different from liquids and gases like water and air, solids do not always require any container to shape them, because they can make a shape by themselves. Therefore, we often presume that most substances around us are solids. Generally speaking, the following three fundamental conditions of materials and substances must be fulfilled for practical use.
- It possesses characteristic properties or functions that cannot be found for the other materials.
- It is harmless for human beings and natural environments.
- The raw materials must be abundantly and readily obtainable and they can be produced with reasonable price.
Now, let's look at how TiO2 fulfills these conditions as a useful and practical material in our daily lives.
Every material consists of various atoms which are categorized by elements. The number of elements on Earth is about 100. The major material of usual chemical substances mostly consists of from one to three elements. Taking into account the number of the combination of these elements, a variety of solids would be formulated. Now the question is what are the states of the atomic nuclei and electrons in the solid when we remember that an atom consists of a positively charged nucleus and several negatively charged electrons surrounding the nucleus. Because the atomic mass is mostly determined by the weight of the nuclei, the state of substance changes when the nucleus moves. On the other hand, electrons are very light and play a role to connect distinct nuclei. But they cannot always move around within the solid. The arrangement of the atoms determines the character of the solid. In a crystalline state, the atoms are arranged regularly. Otherwise, the state is called non-crystalline (amorphous). In the crystalline form, the atomic defect disappears and the distance of electron movement becomes prolonged. On the other hand, amorphous materials like glass are randomly directed and are then expected to easily form a smooth film. According to the intrinsic characteristics and formulation, the individual functions of the materials emerge to be utilized as substances which possess various mechanical, electric, magnetic, and optical functions. To improve these characteristics, novel materials have been designed and developed.
1.2 Titanium Dioxide TiO2 – a Representative Photocatalyst
A representative photocatalyst, titanium dioxide (TiO2) is a white inorganic compound, which is stable and non-toxic. It is characterized by the extremely large refractive index, dielectric constant, and insulation resistance. TiO2 has been utilized widely as a pigment because the natural abundance of Ti is as high as ninth among the existing elements on Earth and the price is relatively low. It is used for delustering white cloth such as shirts, fillers for papers, plastics, paints, and white rubber. However, few people know that an average person usually wears several grams of titanium oxide.
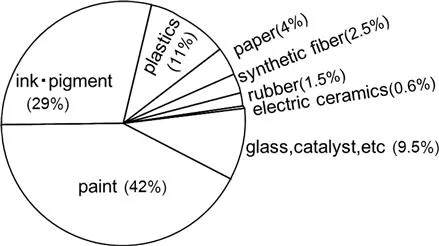
The recent trends of the applications of TiO2 are shown in Figure 1.1. Nowadays, 89% of TiO2 are used as pigments for many products by taking advantage of the considerably high refractive index. In addition, it is used for capacitors as electric ceramics by exploiting the high dielectric constant. Recently, as a raw material of glass and barium titanate, it has been utilized for capacitors and various sensors.
Figure 1.1 Proportion of the use of TiO2. (Japan Titanium Dioxide Industrial Association, Sept. 2010).
The annual global consumption of titanium dioxide is 4 million tons. The amount of the production increases with GNP. It has been recently demonstrated to be useful for our daily life because of the photocatalytic functions caused by the chemical reactions by absorbing UV light contained in sunlight or a fluorescent lamp. The functions have opened up wider applications of TiO2 in various fields, such as environmental clean-up, phototherapy, antifogging, and self-cleaning of mirrors and glasses. Now, let's look at why TiO2 came to be used as a material in the wide field.
We will see the points of TiO2 superior to the other materials. The representative properties of TiO2 and ZnO photocatalysts, such as density (specific gravity), specific heat (thermal capacity), hardness, melting point, refractive index, dielectric constant, bandgap, mobility of the semiconductor carrier, and isoelectric point are shown in Table 1.1. There are two kinds of common crystalline system (rutile and anatase), which differ in the arrangements of atoms and show some different properties. The thermal capacity and density of TiO2 are not particularly different from those of the other materials. However, since the hardness of rutile crystalline is remarkably high, TiO2 is categorized as a hard material. Furthermore, the high melting point and heat resistance are advantages as materials. A more important advantage would be the high refractive index and the relatively wide bandgap, which is the reason why TiO2 has been widely used in daily materials. Besides, although not presented in the table, it is also a prominent characteristic of TiO2 that it is chemically so stable that it cannot be dissolved even in concentrated sulfuric acid, except on heating.
Table 1.1 Properties of TiO2 and ZnO crystals.1–3
| Properties at 298 K | TiO2 (Rutile) | TiO2 (Anatase) | ZnO (Wurtzite) |
| Density (g cm−3) | 4.250 | 3.894 | 5.606 |
| Volume (nm3 molecule−1) | 0.0312 | 0.0341 | 0.0241 |
| Specific heat (J K mol−1) | 55.06 | 55.52 | 43.9 |
| Mohs hardness | 7.0–7.5 | 5.5–6.0 | 4.5 |
| Melting point (°C) | 1840 (decomp.) | trans. to rutile | 1970 (decomp.) |
- Refractive index E//c
- (@nD, 589 nm) E⊥c
| | | |
| Relative permittivity, ε(0) | | 12 | |
| Bandgap energy (eV) | 3.0 (direct) | - 3.8 (direct)
- 3.2 (indirect)
| 3.4 |
| Effective mass (hole) | 20 | 0.8 | 0.24 |
| Mobility (cm2 Vs−1) | 0.1 | 4–20 | 130–205 |
| Isoelectric point | 5.6 | 6.1 | 10.3 |
As shown in Table 1.1, the refractive index of each crystalline form is different. The average value is 2.72 and 2.52 for rutile and anatase, respectively. These values are even larger than that for diamond (2.42), and the largest among the minerals which are transparent for visible light. This means that the incident light is refracted largely at the surface of the solid. As will be described in Chapter 2, the reflection (scattering) of the light on the surface of the solid theoretica...