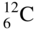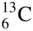Stable Isotope Forensics
Methods and Forensic Applications of Stable Isotope Analysis
Wolfram Meier-Augenstein
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
Stable Isotope Forensics
Methods and Forensic Applications of Stable Isotope Analysis
Wolfram Meier-Augenstein
Über dieses Buch
The number-one guide, internationally, to all aspects of forensic isotope analysis, thoroughly updated and revised and featuring many new case studies
This edition of the internationally acclaimed guide to forensic stable isotope analysis uses real-world examples to bridge discussions of the basic science, instrumentation and analytical techniques underlying forensic isotope profiling and its various technical applications. Case studies describe an array of applications, many of which were developed by the author himself. They include cases in which isotope profiling was used in murder, and drugs-related crime investigations, as well as for pharmaceutical and food authenticity control studies.
Updated with coverage of exciting advances occurring in the field since the publication of the 1 st edition, this 2 nd edition explores innovative new techniques and applications in forensic isotope profiling, as well as key findings from original research. More than a simple update, though, this edition has been significantly revised in order to address serious problems that can arise from non-comparable and unfit-for-purpose stable isotope data. To that end, Part II has been virtually rewritten with greater emphasis now being placed on important quality control issues in stable isotope analysis in general and forensic stable isotope analysis in particular.
- Written in a highly accessible style that will appeal to practitioners, researchers and students alike
- Illustrates the many strengths and potential pitfalls of forensic stable isotope analysis
- Uses recent case examples to bridge underlying principles with technical applications
- Presents hands-on applications that let experienced researchers and forensic practitioners match problems with success stories
- Includes new chapters devoted to aspects of quality control and quality assurance, including scale normalisation, the identical treatment principle, hydrogen exchange and accreditation
Stable Isotope Forensics, 2 nd Edition is an important professional resource for forensic scientists, law enforcement officials, public prosecutors, defence attorneys, forensic anthropologists and others for whom isotope profiling has become an indispensable tool of the trade. It is also an excellent introduction to the field for senior undergraduate and graduate forensic science students.
"All students of forensic criminology, and all law enforcement officers responsible for the investigation of serious crime, will want to study this book. Wolfram highlights the value, and future potential, of Stable Isotope Forensics as an emerging powerful tool in the investigation of crime."
—Roy McComb, Deputy Director, Specialist Investigations, National Crime Agency (NCA), UK
" A single author text in these days is rare and the value of this book lies in the dedication and experience of the author which is evident in the clarity of prose, the honest illustration of evidence and the realistic practical application of the subject - it makes this a text of genuine scientific value."
— Prof Dame Sue Black, PhD, DBE, OBE, FRSE, Leverhulme Research Centre for Forensic Science, University of Dundee, UK
The book provides an excellent, vivid and comprehensible introduction into the world of stable isotope science and analytics. Compared to the first edition, the aspects of quality control and assurance in the analysis of stable isotopes in general, and forensic application in particular, are now taking much more room. This allows the book to serve the target groups: students, academic professionals and practitioners, and serves as a solid resource of basic and applicable information about the strengths and potential pitfalls of the application of stable isotope signatures. The present high-quality book shows the great potential of stable isotopes and is a must for everyone interested in isotope forensics.
M.E. Böttcher & U. Flenker, Isotopes in Environmental and Health Studies, January 2018. A list of errata is available at http://booksupport.wiley.com
Häufig gestellte Fragen
Information
Part I
How it Works
Chapter I.1
What are Stable Isotopes?