
eBook - ePub
Antibody-Drug Conjugates
Fundamentals, Drug Development, and Clinical Outcomes to Target Cancer
Kenneth J. Olivier, Sara A. Hurvitz, Kenneth J. Olivier, Sara A. Hurvitz
This is a test
Buch teilen
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Antibody-Drug Conjugates
Fundamentals, Drug Development, and Clinical Outcomes to Target Cancer
Kenneth J. Olivier, Sara A. Hurvitz, Kenneth J. Olivier, Sara A. Hurvitz
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
Providing practical and proven solutions for antibody-drug conjugate (ADC) drug discovery success in oncology, this book helps readers improve the drug safety and therapeutic efficacy of ADCs to kill targeted tumor cells.
•Discusses the basics, drug delivery strategies, pharmacology and toxicology, and regulatory approval strategies
•Covers the conduct and design of oncology clinical trials and the use of ADCs for tumor imaging
•Includes case studies of ADCs in oncology drug development
•Features contributions from highly-regarded experts on the frontlines of ADC research and development
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Antibody-Drug Conjugates als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Antibody-Drug Conjugates von Kenneth J. Olivier, Sara A. Hurvitz, Kenneth J. Olivier, Sara A. Hurvitz im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Medicine & Pharmacology. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
Part I
What is an Antibody–Drug Conjugate
Chapter 1
Typical Antibody–Drug Conjugates
John M. Lambert
ImmunoGen, Inc., Waltham, MA, USA
1.1 Introduction
1.1.1 A Simple Concept
Ever since cancer patients were first treated with cytotoxic agents with the goal of eradicating the tumor tissue, oncologists have looked to widen the therapeutic window for these agents. The goal of combination chemotherapy, pioneered by Emil “Tom” Frei and others [1], was to increase antitumor efficacy of cytotoxic drug therapy, without substantially increasing overall toxicity to the patient, by using agents with nonoverlapping dose-limiting toxicities. However, such modalities have proven only partially effective at the maximum achievable doses, limited by the severe side effects of the cytotoxic agents used. Attaching cytotoxic effector molecules to an antibody to form an antibody–drug conjugate (ADC) provides a mechanism for the selective delivery of the cytotoxic payload to cancer cells via the specific binding of the antibody moiety to cancer-selective cell surface molecules. This simple concept was thought to be a particularly attractive solution to the challenge of finding a way to increase the therapeutic window of the cytotoxic agent (Figure 1.1). Furthermore, conjugation of a small molecular weight cytotoxic agent to a large hydrophilic antibody protein is expected to restrict penetration of the cytotoxic compound across cellular membranes of antigen-negative normal cells, providing an additional mechanism by which the therapeutic index of the small molecule cytotoxin is widened, beyond that of targeted delivery. Thus, from the perspective of a medicinal chemist, an ADC is a prodrug that can only be activated within tumor cells and is excluded from normal cells by virtue of conjugation to a protein. In addition, giving the in vivo distribution properties of an antibody to the small molecular weight cytotoxic agent has the potential to reduce its systemic toxicity.
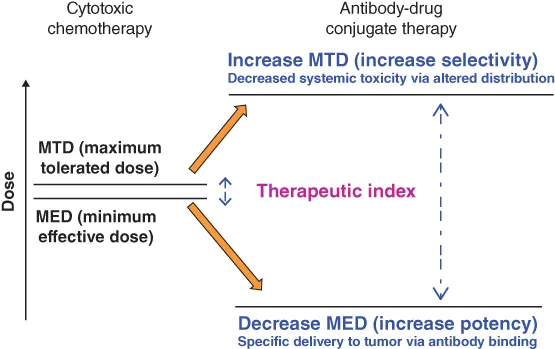
Figure 1.1 Increasing the therapeutic index of cytotoxic drugs by conjugation to antibodies.
1.1.2 Turning Antibodies into Potent Anticancer Compounds
There is another way to look at the simple concept of an ADC. Ever since the advent of monoclonal antibody technology [2], a focus of cancer research has been to develop antibodies for anticancer therapy. Indeed, four monoclonal antibodies, rituximab, trastuzumab, cetuximab, and bevacizumab, are among the most commercially successful anticancer drugs [3]. However, many more antibodies to a variety of target antigens have been tested, both in preclinical studies and in clinical trials, and have proven to have insufficient anticancer activity to be developed as therapeutic agents. In general, the immunologic mechanisms for killing malignant cells induced upon binding of antibodies to cell surface antigens present in cancers appear to be insufficient to affect significant reduction in tumor cell burden in most instances. Thus, providing an additional killing mechanism to such anticancer antibodies via conjugation to cytotoxic agents was thought to be a solution to their lack of potency. From the perspective of an immunologist, enhancing antibody activity by creating ADCs was one approach to be able to fully exploit the full potential of their exquisite specificity toward tumor cells [4–6].
1.1.3 What is a Typical ADC and How Does it Act?
A typical ADC consists of several molecules of a potent cytotoxic agent (generally in the range of two to six molecules per antibody molecule on average), which are linked covalently to side chains of particular amino acid residues of a monoclonal antibody (Figure 1.2). The chosen linker chemistry should be sufficiently stable during in vivo circulation in the bloodstream so that the payload stays linked to the antibody during the time it takes for the antibody to distribute into tissues, yet must allow release of an active cytotoxic compound once the ADC is taken up by cancer cells within tumor tissue. Once at the tumor, the antibody component of the ADC binds specifically to its target antigen on cancer cells; in the case of a typical ADC, the cytotoxic payload is liberated after internalization of the antibody–antigen complex and routing to the relevant intracellular compartment for release of an active cytotoxic compound from the ADC (Figure 1.2).
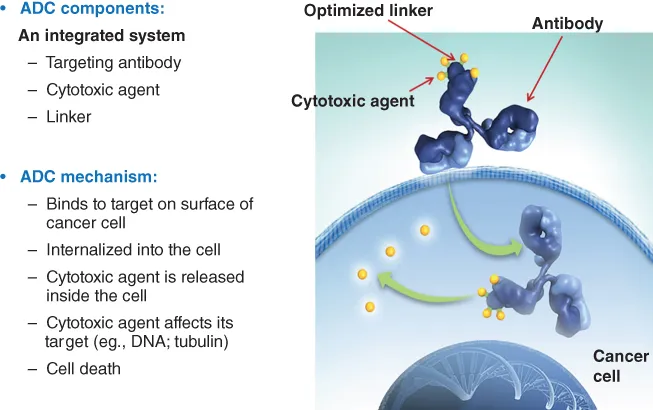
Figure 1.2 The components of an ADC and its mechanism of action.
1.1.4 Simple Concept, but Not So Simple to Execute
The earliest notion in the field of ADC research was that conjugation to specific monoclonal antibodies was a way to widen the therapeutic window of existing chemotherapeutic drugs, such as the vinca alkaloids [7], and doxorubicin [8], following on from the early attempts to provide specificity to cytotoxic drugs by conjugation to serum immunoglobulins [9]. However, despite the early optimism generated by some of the preclinical results [8], the results of clinical trials of such conjugates were disappointing [10–12]. During the 1980s, increased knowledge of the biodistribution properties of monoclonal antibodies based on clinical dosimetry measurements with radiolabeled antibodies pointed to one explanation for such disappointing results. It was found that the amount of antibody that could be localized to a solid tumor 24 h after administration, a time corresponding approximately to the peak delivered concentration, was only about 0.01% of the injected dose of antibody per gram of tumor tissue for a range of different antibodies, to a variety of targets in patients with a variety of tumor types [13]. Thus, it was reasoned that the lack of clinical benefit from ADCs made with conventional chemotherapeutic drugs was that not enough of these agents could be localized at the tumor via antibody-mediated delivery to have an antitumor effect. The use of these only moderately cytotoxic compounds as payloads for ADCs was at least one of the barriers to the successful execution of the ADC concept. The idea that conventional chemotherapeutic drugs were not potent enough to serve as payloads for ADCs has guided much of the subsequent research in the field [4–6].
1.2 The Building Blocks of a Typical ADC
All three parts of an ADC, the antibody, the cytotoxic payload, and the linker chemistry that joins them together, are important in designing an ideal ADC. The design goal is to add the potent tumor cell-killing mechanism afforded by the payload, while retaining all the favorable properties of the antibody in terms of in vivo pharmacokinetics and biodistribution, together with any intrinsic biologic activity and immunologic properties. It is beyond the scope of this chapter to discuss the properties of the cell surface target molecule, but suffice to say that selecting the right target, and matching the design of the ADC to the properties of the target, is vital to the creation of an effective therapeutic agent.
1.2.1 The Antibody
The first monoclonal antibodies used in ADCs and also in immunotoxins – antibodies conjugated to potent protein toxins such as derivatives of ricin, or diphtheria toxin [14] – were murine antibodies. However, apart from other limitations, such conjugates proved to be immunogenic in humans [10]. The advent of chimerization and a variety of humanization techniques (CDR grafting, resurfacing) for rendering murine antibodies less immunogenic or nonimmunogenic in humans [15], and the methods for cloning of human immunoglobulin genes into a variety of organisms, such as transgenic animals, bacteriophage, or yeast, for the generation of fully human antibodies [16–18], have largely addressed this problem (Figure 1.3), as has been generally borne out by the recent clinical experience with ADCs [19]. Of the 51 ADCs currently in clinical trials, at least two utilize chimeric antibodies, including the approved ADC, brentuximab vedotin, while for the other ADCs, antibody usage is, where known, fairly evenly split between humanized antibodies and fully human antibodies. Several of the humanizations were done by the method of variable domain resurfacing [15], for example, the anti-CanAg antibody, cantuzumab, utilized in the first maytansinoid ADC (cantuzumab mertansine) to enter into clinical trials [20]. Recently, however, the World Health Organization decided to alter criteria for providing generic names to antibodies, resulting in the confusing situation of many humanized antibodies being given names bearing the suffix of a chimeric antibody (“-ximab”), for example, the an...