
eBook - ePub
Eosinophils in Allergy and Inflammation
Gerald J. Gleich, Gerald J. Gleich
This is a test
Compartir libro
- 528 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
eBook - ePub
Eosinophils in Allergy and Inflammation
Gerald J. Gleich, Gerald J. Gleich
Detalles del libro
Vista previa del libro
Índice
Citas
Información del libro
This work presents the proceedings of a conference held at Adare Manor, County Limerick, Ireland. It provides an updated, in-depth review of the biological role of eosinophils in allergic diseases, summarizing basic knowledge of these unique cationic proteins. The book features an annotated discussion of the conference's post-presentation question-and-answer session.
Preguntas frecuentes
¿Cómo cancelo mi suscripción?
¿Cómo descargo los libros?
Por el momento, todos nuestros libros ePub adaptables a dispositivos móviles se pueden descargar a través de la aplicación. La mayor parte de nuestros PDF también se puede descargar y ya estamos trabajando para que el resto también sea descargable. Obtén más información aquí.
¿En qué se diferencian los planes de precios?
Ambos planes te permiten acceder por completo a la biblioteca y a todas las funciones de Perlego. Las únicas diferencias son el precio y el período de suscripción: con el plan anual ahorrarás en torno a un 30 % en comparación con 12 meses de un plan mensual.
¿Qué es Perlego?
Somos un servicio de suscripción de libros de texto en línea que te permite acceder a toda una biblioteca en línea por menos de lo que cuesta un libro al mes. Con más de un millón de libros sobre más de 1000 categorías, ¡tenemos todo lo que necesitas! Obtén más información aquí.
¿Perlego ofrece la función de texto a voz?
Busca el símbolo de lectura en voz alta en tu próximo libro para ver si puedes escucharlo. La herramienta de lectura en voz alta lee el texto en voz alta por ti, resaltando el texto a medida que se lee. Puedes pausarla, acelerarla y ralentizarla. Obtén más información aquí.
¿Es Eosinophils in Allergy and Inflammation un PDF/ePUB en línea?
Sí, puedes acceder a Eosinophils in Allergy and Inflammation de Gerald J. Gleich, Gerald J. Gleich en formato PDF o ePUB, así como a otros libros populares de Medicine y Immunology. Tenemos más de un millón de libros disponibles en nuestro catálogo para que explores.
Información
1
Eosinophil Granule Proteins: Structure and Function
Gerald J. Gleich and Randa I. Abu-Ghazaleh
Mayo Clinic and Mayo Foundation, Rochester, Minnesota
Dohn G. Glitz
UCLA School of Medicine, University of California, Los Angeles, California
I. INTRODUCTION
The pioneering studies of white blood cell morphology by Paul Ehrlich established that one granulated leukocyte stained with essentially all acid dyes (and Ehrlich tried over 30 acid dyes), but this same leukocyte was not stained by the basic aniline dyes (1). Because Ehrlich discovered that the acid dye eosin was especially useful for staining this cell, the peripheral blood leukocyte containing the acidophilic granules was named the eosinophil. Ehrlich’s observations presumed the existence of basic charge in the eosinophil, and subsequent observations have abundantly substantiated this presumption. Here, information on the principal cationic eosinophil granule proteins will be reviewed with emphasis on the toxicity of the major basic protein (MBP) and the ability of the eosinophil-derived neurotoxin (EDN) and the eosinophil cationic protein (ECP) to elicit the neurotoxic reaction in rabbits referred to as the Gordon phenomenon.
II. EOSINOPHIL GRANULE PROTEINS
Analyses of eosinophils from guinea pigs and humans have established the existence of four predominant cationic proteins, referred to as MBP, the eosinophil peroxidase (EPO), EDN, and ECP.
A. Major Basic Protein
MBP is so named because in the guinea pig it accounts for approximately 55% of granule protein, its isoelectric point is >10, and it is proteinaceous in nature (2, 3, 4). Analysis of guinea pig MBP by sodium dodecyl sulfate–polyacrylamide gel electrophoresis revealed a molecular weight of approximately 11,000 and showed that the molecule is rich in arginine and has a marked propensity to polymerize on the basis of the formation of disulfide bonds. Analyses of human MBP showed that it has similar properties (5). Detailed studies of human MBP indicated that it consists of a single polypeptide chain of 117 amino acids, has a molecular weight of about 14,000, and is rich in arginine with a calculated isoelectric point of 10.9 (6,7). The MBP cDNA specifies the existence of a prepromolecule with a 15-amino-acid leader sequence and a 90-amino-acid prosequence followed by the 117-amino-acid sequence for MBP itself (7,8). The 90-amino-acid pro-portion is markedly enriched in acidic amino acids, especially glutamic acid, and has an isoelectric point of 3.9. When the pro-portion of MBP and the mature protein are combined, one obtains a molecule of 270 amino acids with roughly equal numbers of strongly basic and strongly acidic amino acids and an isoelectric point of 6.2. The balance of charge between the pro-portion and MBP itself suggests that the pro-portion serves to neutralize the toxic properties of MBP. In this view, the proportion would protect the cell from the toxic effects of MBP during the transport of pro-MBP from the Golgi apparatus to the eosinophil granule. Of interest, the cDNA of barley toxin-α-hordothionin, another toxic cationic protein, codes for a nearly neutral precursor (9). Here, the isoelectric point of the barley toxin-α-hordothionin is 9.6 and the isoelectric point of the pro-portion is 3.6 so that the isoelectric point of the entire molecule is 7.6.
The occurrence of a pro-portion in MBP has also been observed in the guinea pig (10,11). Two types of guinea pig MBP, MBP-1 and MBP-2, have been isolated and partially sequenced. Analyses of cDNA indicated that both types show a prepro-MBP with three domains consisting of a signal peptide, an acidic proportion, and mature MBP. Whereas guinea pig MBP-1 and MBP-2 are quite similar and resemble human MBP, the pro-portions of the molecule are not very homologous to the human propiece despite similar pI values. These results indicate that the synthesis of MBP proceeds through a promolecule with a markedly acidic pro-portion in both humans and guinea pigs. Furthermore, while the MBP portions are quite homologous, the pro-portions are less so, suggesting that anionic charge and not sequence homology is critical.
Information regarding MBP localization and MBP function is summarized in Table 1 and reviewed in detail in Ref. 12. Briefly, MBP is localized to the eosinophil granule; it is also present in basophils and placental X cells (13). MBP is a potent toxin and kills parasites, both helminths and protozoa, bacteria, and mammalian cells. It causes histamine release from basophils and rat mast cells, activates neutrophils and platelets, and causes bronchoconstriction and bronchial hyperreactivity when instilled into the lungs of monkeys.
Table 1 Some Properties of Cationic Human Eosinophil Granule Proteins and Their Encoding cDNA and Genes
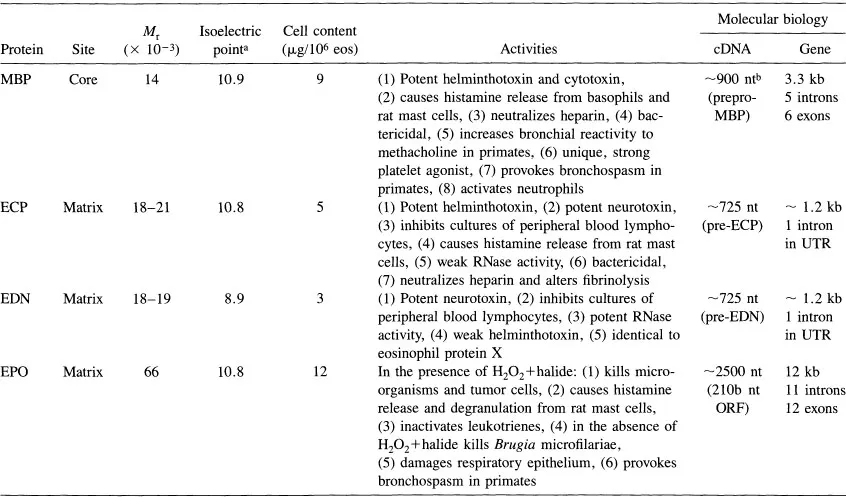
aCalculated from amino acid sequences deduced from the cDNAs.
bnt = nucleotides; kb = kilobases; ORF = open reading frame; UTR = untranslated region; see Ref. 12 for references concerning the molecular biology of these proteins and their genes.
Source: Used with permission from Ref. 12.
B. Eosinophil Peroxidase
EPO differs from neutrophil myeloperoxidase (MPO) in its physicochemical properties and in its deduced amino acid sequence (12). Human EPO consists of two subunits, a heavy chain with Mr 54–58 × 103 and a light chain with Mr 10.5–15.5 × 103 (Table 1). EPO cDNA has been cloned and consists of an open reading frame of 2106 nucleotides coding for a 381-base-pair prosequence, a 333-base-pair sequence coding for the EPO light chain, a 1392-base-pair sequence coding for the EPO heavy chain, and a 452-base-pair untranslated 3′ region. EPO belongs to the peroxidase multigene family, including EPO, MPO, thyroid peroxidase, and lactoperoxidase.
When armed with H2O2 and a halide, such as Br−, EPO generates HOBr, a potent oxidant. Similar reactions occur with I− and Cl−, a...