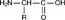Abstract:
This chapter provides a brief overview of the structural characteristics and functional properties of proteins that are used in food products. It highlights the mechanisms responsible for the key functional behaviour of both linear and globular proteins which are to form gels and to stabilise oil-in-water emulsions. It also notes the growing interest in the use of proteins as edible films to enhance the shelf life of fruit and vegetables and in biodegradable packaging. The chapter also introduces the scope of the book and the contributions provided by leading experts in this field of research covering a broad range of food proteins which are derived from various sources including, animal, botanical, macro-algal and micro-organisms.
1.1 Introduction
Proteins are present in all living things and have a key role in many biological processes such as cell signalling, cell adhesion and the immune response. They may also have a structural or mechanical function in, for example, the muscles and connective tissue of animals and the cell walls of plants. It is now recognised that proteins represent a valuable renewable resource and a number of proteins are processed on an industrial scale for application in a range of areas including food, cosmetics, pharmaceuticals, medicine, adhesives, packaging, coatings, etc. This book provides an overview of the source, structure, properties of commercially important food proteins and concentrates, in particular with their application as food additives and food ingredients. A list of common food proteins is presented in Table 1.1.
Table 1.1
Source of common food proteins
| Source | |
| Animal | Muscle proteins
Blood proteins
Proteins in the connective tissue
Milk proteins
Egg proteins |
| Botanical | Cereals
Wheat, corn, barley, oats, rice
Legumes and pulses
Peas, soybeans, lupins, lentils
Tubers
Potato
Oil seeds
Rapeseed, cottonseed, peanut |
| Macro-algae | Green and blue-green seaweed
Spirulina, Anabaena, Nostoc, Ulva, Enteromorpha |
| Micro-organisms | Fungi
Mycoprotein |
1.2 Structure of protein
There are 20 L-α-amino acids which are the building blocks of all proteins. Each amino acid contains a primary amine and carboxylic acid group with the general formula:
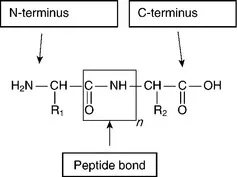
The R group differs for the various amino acids and can impart polar, non-polar, anionic or cationic characteristics. The amino acid units are linked together through a peptide bond to form a polypeptide chain of a characteristic length as illustrated below:
The free OH group at the C-terminus is available to form further peptide links and proteins consisting of 15–10,000 amino acids are known. Since proteins contain both cationic and anionic charges due to the presence of ionisable groups, notably amine and carboxyl, they have a characteristic isoelectric point which corresponds to the pH at which the molecules have a net zero charge.
The primary structure of proteins is defined by the characteristic sequence of amino acids of the polypeptide chain. Certain amino acids within the chain can give rise to local secondary structures such as the alpha helix and beta sheet and a variety of such secondary structures can exist within a single protein molecule. While some protein molecules adopt linear conformations, others fold to varying extents to form more globular structures and the overall shape of the protein, which is referred to as the tertiary structure, is stabilised by a range of interactions including hydrogen bonding, disulfide bonding and salt bridges. Hydrophobic amino acids tend to reside in the interior of globular proteins with hydrophilic amino acids at the periphery. Linear proteins function as structural elements, such as in the connective tissue of animals. The polypeptide chains are arranged in parallel forming long fibres; examples include collagen found in tendons, cartilage and bone, and keratin in hair, skin and nails. Globular proteins are usually soluble in an aqueous environment and are involved, for example, in transport processes or dynamic functions in the cell.
1.3 Functional properties of proteins
Whilst it is clear that proteins have a major function in many biological processes, they also have a key role as food additives and ingredients. As will become clear on reading the various chapters in this book, a common feature is the ability of many proteins to form gels, to stabilise emulsions and foams and to form films.
1.3.1 Gelation
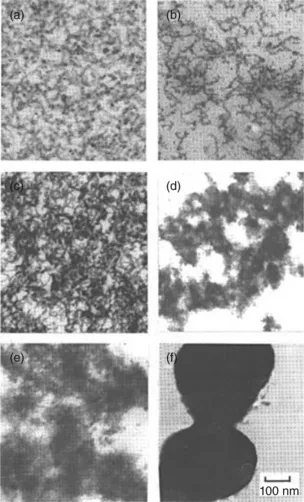
Globular proteins will normally unfold on heating and at sufficiently high concentrations the denatured chains will aggregate to form thermally irreversible gels. The properties of the gels will depend on a number of factors including the degree of unfolding of the protein chains and the extent and kinetics of chain aggregation. Denaturation can also occur at extremes of solution pH and ionic strength. Linear association of the denatured molecules leads to the formation of uniform finely stranded three-dimensional network structures. Association is likely to be driven by interaction between hydrophobic domains along the protein chain which become exposed when the molecules unfold. When the electrostatic charge on the protein chains is significantly reduced, micron-sized protein aggregates are formed, which then associate to form coarse network structures. Transmission electron micrographs of a range of heat-set globular protein gels are presented in Fig. 1.1. The micrographs show the variations in microstructure that can be obtained for different proteins under different solution conditions.
Fig. 1.1 Transmission electron micrographs of: (a) 10% beta lactoglobulin at pH 7; (b) 15% soy glycinin at pH 3; (c) 10% alpha lactalbumin at pH 7; (d) 15% alpha chymotrysin at pH 3; (e) 10% alpha lactalbumin pH 7, 100 mM NaCl; (f) 10% BSA coagulate pH 5.1 [reproduced from A.H. Clark ‘Gelation of globular proteins’ in Functional properties of food macromolecules S.E. Hill, D.A. Ledward and J.R. Mitchell eds Aspen Publishers Inc. Maryland USA 1998 p 77].
In contrast to globular proteins, gelatine, which is a fibrous protein derived from collagen, is able to form thermally reversible gels. Collagen is an extracellular protein present in the bones, skin and connective tissue of humans, animals and fish and adopts a triple helical conformation in the native state. However, in the production of gelatine, the collagenous materials are treated with acid or alkali and the collagen molecules denature producing the heterogeneous material referred to as gelatine. In solution the gelatine molecules adopt a disordered conformation at high temperatures but on cooling to approximately 25°C they undergo a thermally reversible coil-helix transition and the molecules partially reform the collagen triple helical structure. The stiff helical chains will then self-associate to form a three-dimensional gel structure. The degree of helical content and overall rheological properties will be dependent on the solvent conditions and on the rate of cooling. When the gel is re-heated, the association is disrupted and the molecules adopt a disordered structure and the gel melts. Gel melting is usually observed at approximately 37°C and the reason that the melting temperature is greater than the setting temperature is that the helices must disaggregate before the helix-coil transition can occur.
1.3.2 Interfacial properties
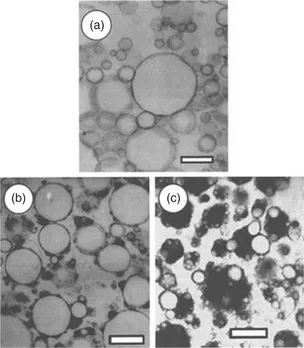
Proteins, particularly those derived from milk and eggs, are commonly used to stabilise oil-in-water emulsions and foams because they are able to adsorb at the oil-water and air-water interfaces. Figure 1.2 shows transmission electron micrographs of various emulsions stabilised by casein. Figure 1.2(a) shows a thin layer of sodium caseinate at the interface of soya oil emulsion droplets and Figs 1.2(b) and 1.2(c) show the attachment of micellar casein (dark areas) at the oil-water interface for homogenised milk samples. The surface activity of different proteins will be a function of their molecular size and conformation. The amino acid composition will control the overall amphiphilic characteristics and the protein’s ability to adsorb at interfaces. During emulsification the role of the protein is to adsorb onto the newly created surface of the oil droplets and prevent droplet aggregation and coalescence. To this end smaller protein molecules are expected to be more effective, since they are able to diffuse to the surface at a faster rate. Larger protein molecules, however, are likely to provide more points of contact and increase the overall energy of adsorption. For globular proteins the ability to unfold at the interface and expose the hydrophobic amino acid groups to facilitate adsorption is a key factor. In addition solvent quality will be important and it is expected that adsorption would be greatest under poor solvent conditions at pH values close to the isoelectric point. Figure 1.3 shows the adsorption isotherms for egg white protein adsorbing onto limonene at pH 3.5 and 7.5. It is noted from the adsorption plateau that the maximum amount adsorbed (corresponding to complete coverage of the droplet surface) is ~ 1–1.5 mg m–2 and also that from the initial slope of the isotherm that the adsorption is low affinity. The difference in the amount adsorbed at the two pHs can be attributed to differences in the net electrostatic charges on the proteins and/or differences in protein conformation. These observations are typical for protein adsorption generally.
Fig. 1.2 Transmission electron micrographs of (a) a soya oil emulsion stabilised by sodium caseinate, (b) and (c) micellar casein adsorbing at the interface of fat...