15.1 Background
The treatment of human immunodeficiency virus (HIV) infection has improved significantly after the introduction of combination antiretroviral therapy (cART), defined as use of a minimum of three active antiretroviral agents, which has resulted in reduced incidence of opportunistic infections and near-normal life expectancy [1,2]. Recent advances in ART include reduced pill burden due to increased availability of fixed-dose combination medications, as well as improved tolerability to newer antiretroviral drugs [1]. However, despite these advances, no cure has been found for HIV infection, and patients experience a multitude of physical symptoms as well as psychosocial issues, including stigma, isolation, and depression. Individuals with HIV also have higher rates of non-acquired immune deficiency syndrome (AIDS)–related disorders, including cardiovascular, kidney, and liver diseases; neurologic impairment; and certain cancers [3]. Due to the chronic nature of HIV infection and related conditions and their impact on quality of life, patients with HIV often seek complementary and alternative therapies.
On average, approximately 60% of patients with HIV report using complementary and alternative medicine (CAM), most commonly agents such as vitamins, herbs, and supplements [4–8]. Studies have shown that patients with HIV primarily use CAM as a complementary measure rather than as an alternative to antiretroviral therapy. The most common reasons for using CAM reported by patients with HIV are to increase energy level, to boost immunity, and to relieve side effects of antiretrovirals [4,6,8]. Patients have also reported using CAM to self-manage health or to gain a sense of control, attempt to normalize health status, or find wellness, as well as follow cultural values [9]. Demographic correlates of CAM use most commonly include Caucasian race compared with other races, male homosexuality compared with other sexual orientations, female gender, higher levels of education, and higher incomes [8–10]. HIV-related predictors of CAM use include longer duration of disease and ART, using a higher number of medications, and the presence of side effects of antiretrovirals [8,9]. Although most studies found that patients who used CAM did not reject conventional medicine [11–15], some studies have noted that CAM use is associated with nonadherence or the decision to not initiate ART [16,17].
Although there is a perception that natural health products (NHPs) are “natural” and therefore have low risks, the use of NHPs may be particularly problematic in patients with HIV due to the risk of NHP–drug interactions and overlapping toxicities, which can lead to treatment failure. One study found that of the 293 patients with HIV included in the study, 10% were advised to stop taking CAM due to concerns about serious drug interactions with ART or the adverse effects of CAM; 15% were advised to use CAM with caution due to potential interactions [5]. However, health care professionals do not yet routinely ask patients about use of NHPs, and studies have found that patient disclosure about CAM use varies considerably, between 38% and 90% [8]. This chapter provides an overview of the mechanisms of drug interactions between NHPs and antiretroviral drugs, pharmacokinetic and pharmacodynamic interactions, and recommendations for screening patients for potential interactions between antiretrovirals and NHPs. For the purposes of this chapter, NHPs include vitamin and minerals, herbal remedies, probiotics, traditional medicine, and homeopathic medicines, as well as other products such as amino acids and essential fatty acids [18].
15.2 Mechanisms of Drug Interactions with Antiretrovirals
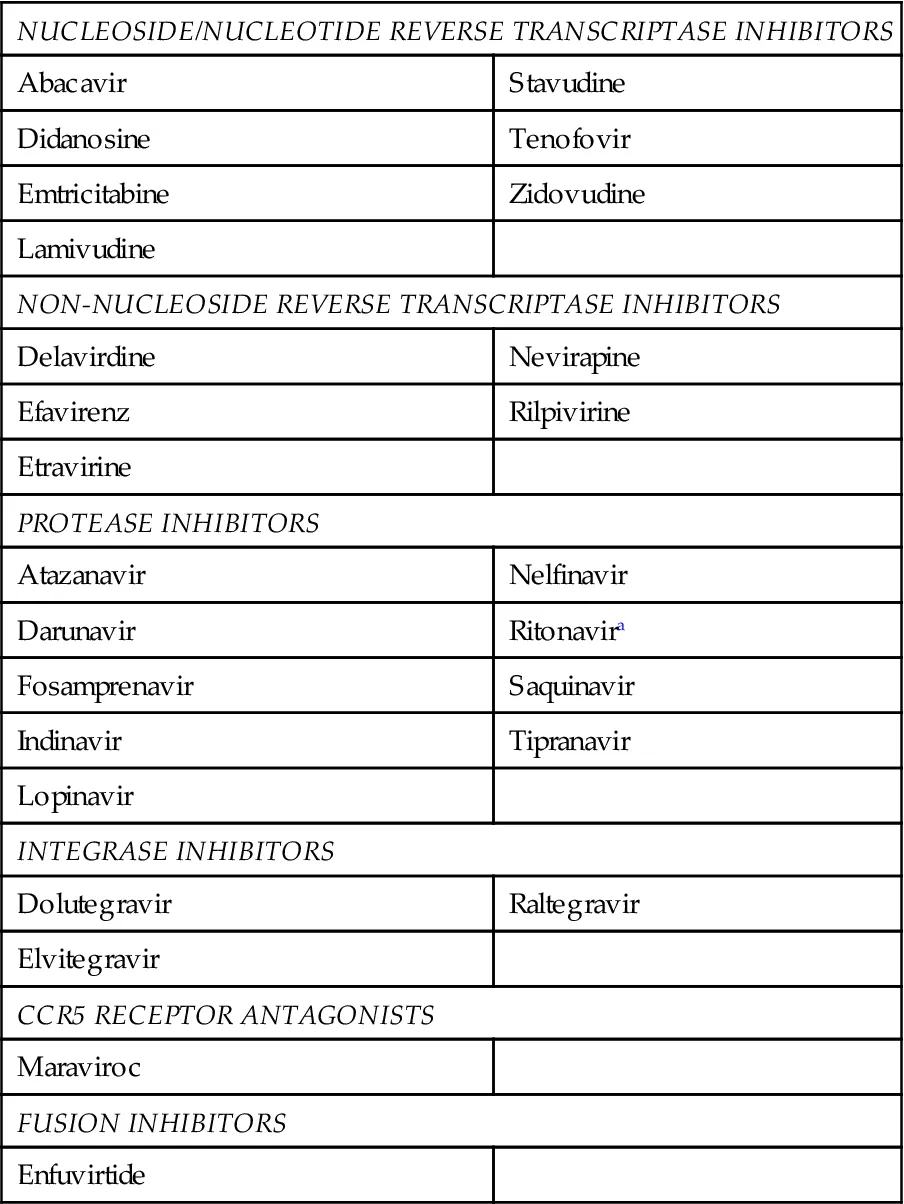
Depending on the mechanism, drug interactions can be classified as either pharmacokinetic or pharmacodynamic. Pharmacokinetic interactions alter the absorption, distribution, metabolism, or excretion of another agent, whereas pharmacodynamic interactions lead to additive, synergistic, or antagonistic responses to drug therapy [19]. Antiretroviral drugs are frequently prone to pharmacokinetic drug–drug interactions as a result of the altered metabolism of the antiretroviral, the co-administered drug, or both or through the effects on transport proteins in the liver or intestine [19]. Drug interactions can result in an increased or decreased concentration of the antiretroviral drug, the co-administered agent, or both, resulting in either increased toxicity or therapeutic failure. Therapeutic failure of antiretroviral drugs is particularly worrisome, as it can lead to development of drug resistance, resulting in mutations and limiting future antiretroviral treatment options. There are more than 20 approved antiretroviral drugs that belong to six different medication classes [1] (Table 15.1). The non-nucleoside reverse transcriptase inhibitor (NNRTI) and protease inhibitor (PI) drug classes, chemokine receptor-5 antagonists (e.g., maraviroc), and integrase inhibitors (elvitegravir) are primarily metabolized by CYP3A4 in the liver. PIs are also substrates for P-glycoprotein and are inhibitors of CYP3A4, with ritonavir being the most potent [19]. Ritonavir is frequently used in low doses (100–200 mg/day) to “boost” the concentrations of co-administered PIs [1]. Ritonavir also inhibits CYP2D6 in the liver and induces CYP2C19, CYP2C9, and CYP1A2, increasing the risk for drug–drug interactions [19]. The NNRTIs efavirenz, nevirapine, and etravirine are inducers of CYP3A4; efavirenz and nevirapine are also inducers of CYP2B6 [19]. In addition, efavirenz and etravirine are inhibitors of CYP2C9 and CYP2C19. The integrase inhibitors, raltegravir and dolutegravir, have a lower potential for interactions, as these agents are primarily metabolized by uridine diphosphate glucuronosyltransferase (UGT) 1A1. Dolutegravir is also a substrate of CYP3A4 and P-glycoprotein in vitro [20]. Elvitegravir, however, is co-formulated with cobicistat, a potent inhibitor of CYP3A4, which is used to boost concentrations of elvitegravir but is also prone to interactions with other co-administered drugs [21]. Nucleoside reverse transcriptase inhibitors (NRTIs) are primarily renally cleared, and thus, pharmacokinetic interactions are less likely.
Table 15.1
Antiretroviral Agents
| NUCLEOSIDE/NUCLEOTIDE REVERSE TRANSCRIPTASE INHIBITORS |
| Abacavir | Stavudine |
| Didanosine | Tenofovir |
| Emtricitabine | Zidovudine |
| Lamivudine | |
| NON-NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS |
| Delavirdine | Nevirapine |
| Efavirenz | Rilpivirine |
| Etravirine | |
| PROTEASE INHIBITORS |
| Atazanavir | Nelfinavir |
| Darunavir | Ritonavira |
| Fosamprenavir | Saquinavir |
| Indinavir | Tipranavir |
| Lopinavir | |
| INTEGRASE INHIBITORS |
| Dolutegravir | Raltegravir |
| Elvitegravir | |
| CCR5 RECEPTOR ANTAGONISTS |
| Maraviroc | |
| FUSION INHIBITORS |
| Enfuvirtide | |
aLow-dose ritonavir and cobicistat are used as pharmacokinetic boosters for select antiretrovirals.