Group Theory for Chemists
Fundamental Theory and Applications
Kieran C Molloy
- 232 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
Group Theory for Chemists
Fundamental Theory and Applications
Kieran C Molloy
Información del libro
The basics of group theory and its applications to themes such as the analysis of vibrational spectra and molecular orbital theory are essential knowledge for the undergraduate student of inorganic chemistry. The second edition of Group Theory for Chemists uses diagrams and problem-solving to help students test and improve their understanding, including a new section on the application of group theory to electronic spectroscopy.Part one covers the essentials of symmetry and group theory, including symmetry, point groups and representations. Part two deals with the application of group theory to vibrational spectroscopy, with chapters covering topics such as reducible representations and techniques of vibrational spectroscopy. In part three, group theory as applied to structure and bonding is considered, with chapters on the fundamentals of molecular orbital theory, octahedral complexes and ferrocene among other topics. Additionally in the second edition, part four focuses on the application of group theory to electronic spectroscopy, covering symmetry and selection rules, terms and configurations and d-d spectra.Drawing on the author's extensive experience teaching group theory to undergraduates, Group Theory for Chemists provides a focused and comprehensive study of group theory and its applications which is invaluable to the student of chemistry as well as those in related fields seeking an introduction to the topic.
- Provides a focused and comprehensive study of group theory and its applications, an invaluable resource to students of chemistry as well as those in related fields seeking an introduction to the topic
- Presents diagrams and problem-solving exercises to help students improve their understanding, including a new section on the application of group theory to electronic spectroscopy
- Reviews the essentials of symmetry and group theory, including symmetry, point groups and representations and the application of group theory to vibrational spectroscopy
Preguntas frecuentes
Información
Symmetry
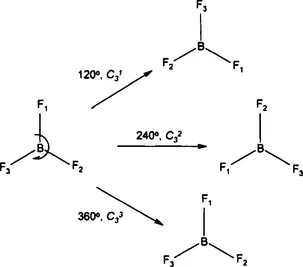
1.1 SYMMETRY
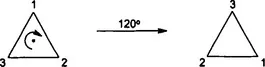
1.1.1 Rotations and Rotation Axes