![]()
SECTION I
Biophysical Chemistry, Electrochemistry, Metabolism, Second Messengers, and Ultrastructure
Outline
Chapter 1: Biophysical Chemistry of Cellular Electrolytes
Chapter 2: The Physiological Structure and Function of Proteins
Chapter 3: Lateral Lipid Domains and Membrane Function
Chapter 4: Ultrastructure of Cells
Chapter 5: Diffusion and Permeability
Chapter 6: Origin of Resting Membrane Potentials
Chapter 7: Gibbs–Donnan Equilibrium Potentials
Chapter 8: Energy Production and Metabolism
Chapter 9: Signal Transduction
Chapter 10: Calcium as an Intracellular Second Messenger: Mediation by Calcium Binding Proteins
![]()
1
Biophysical Chemistry of Cellular Electrolytes
Publisher Summary
Biophysical chemistry is defined as the application of the concepts and methods of physical chemistry to the study of biological systems. Physical chemistry includes physiologically relevant subjects, such as thermodynamics, chemical equilibria and reaction kinetics, solutions and electrochemistry, properties and kinetic theory of gases, transport processes, surface phenomena, and molecular structure and spectroscopy. This chapter discusses the basic concepts of cell physiology by describing certain physicochemical properties of electrolytes and proteins that are relevant to an understanding of the structure and function of cells. All living cells contain proteins, salts, and water enclosed in membrane-bounded compartments. These biochemical and ionic cellular constituents, together with a set of genes, enzymes, substrates, and intermediates, function to maintain cellular homeostasis and enable cells to perform chemical, osmotic, mechanical, and electrical work. Concepts of biophysical chemistry are exemplified by human red blood cells, which lack membrane-bounded intracellular organelles and are devoid of the complexities introduced by intracellular compartments.
I Introduction
Biophysical chemistry may be defined as the application of the concepts and methods of physical chemistry to the study of biological systems. Physical chemistry includes such physiologically relevant subjects as thermodynamics, chemical equilibria and reaction kinetics, solutions and electrochemistry, properties and kinetic theory of gases, transport processes, surface phenomena, and molecular structure and spectroscopy. Many cellular physiological phenomena are best understood by a rigorous and comprehensive understanding of physical chemistry. Physical chemistry texts that specifically emphasize biological applications include those by Tinoco et al. (1985) and Eisenberg and Crothers (1979). Several outstanding monographs on biophysical chemistry are also available: Edsall and Wyman (1958), Tanford (1961), Cantor and Schimmel (1980), van Holde (1985), Silver (1985), and Bergethon and Simons (1990). This chapter and the next will introduce some of the basic concepts of cell physiology by describing certain physicochemical properties of electrolytes and proteins that are relevant to an under-standing of the structure and function of cells.
All living cells contain proteins, salts, and water enclosed in membrane-bounded compartments. These biochemical and ionic cellular constituents, together with a set of genes, enzymes, substrates, and intermediates, function to maintain cellular homeostasis and to enable cells to perform chemical, osmotic, mechanical, and electrical work. Homeostasis means that certain parameters, such as the cellular volume, the intracellular pH, and the intracellular concentrations of salts are maintained relatively constant in resting cells. To understand how cellular homeostasis is achieved, the electrolyte composition and the functions of specific ions in cells will be described first followed by a consideration of some physi-cochemical aspects of water and of solutions of salts and proteins. In this chapter, some concepts of biophysical chemistry will be exemplified by human red blood cells, which lack membrane-bounded intracellular organelles and are thereby devoid of the complexities introduced by intracellular compartments.
II Cell Cations
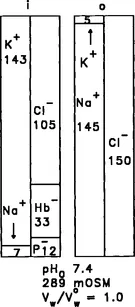
Potassium, sodium, calcium, and magnesium are the predominant biological cations. The ionic composition of the intracellular and extracellular solutions may be de-picted on bar graphs known as Gamblegrams, named after the physiologist J. L. Gamble, who used such diagrams extensively in analyzing the electrolyte balance of the blood and body fluids (Gamble, 1964). A Gamblegram for human red blood cells is shown in Fig. 1. The most abundant ion in the intracellular solution is potassium (K +) at 143 mM, whereas sodium ion (Na+) is only 7 mM (Funder and Wieth, 1966). In the extracellular fluid, Na+ predominates at approximately 145 mM, whereas K+ is only about 5 mM. The Na, K-ATPase, a transport protein located in the plasma membrane, specifically selects K+ from the Na+-rich medium and pumps it against the K+ concentration gradient (and electrochemical gradient) into the cytoplasmic solution. The same ionic pump specifically selects Na+ from the K + -rich cytoplasmic solution and extrudes it from the cell against the Na+ concentration gradient. This coupled active transport of K+ and Na+ uses metabolic energy obtained from the hydrolysis of ATP (for reviews, see Glynn, 1985; Läuger, 1991). At the same time that K+ is actively accumulated in the cell, it is also continually leaking out of the cell through a parallel pathway down its concentration gradient. The same is true for Na+ leaking into the cell. Steady-state distributions of K+ and Na+ are achieved by a balance between active pumping and passive leakage.
FIG. 1 Gamblegram for human red blood cells. The bar graphs illustrate the ionic composition of the intracellular and extracellular solutions, designated by i and o, respectively. Organic phosphates are represented by P−.
A quantitative model of the pump-leak theory that predicted the steady state Na+ and K+ concentrations in high-K+ and low-K+ red blood cells found in genetic variants of sheep was developed by Tosteson and Hoffman (1960).
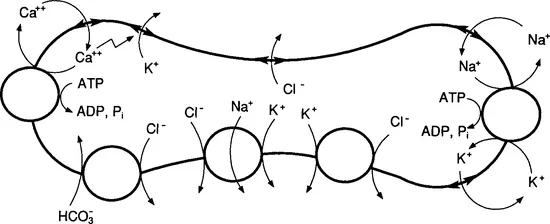
As illustrated in Fig. 2, other transport systems that may also contribute to the steady-state intracellular ionic concentrations of red cells have since been discovered (for review, see Tosteson, 1981). These include Na/K/Cl co-transport and K/Cl cotransport (for review, see Dunham, 1990) and Na/H exchange. The pump-leak theory for red cells has been extended to include some of the effects of these additional cotransport pathways (Milanick and Hoffman, 1986).
FIG. 2 The principal membrane transport systems of human red blood cells. Starting with the Na+/K+ pump and the passive leakage pathways for Na+ and K+ on the right, and proceeding clockwise around the memb...