Introduction
Vitamin K [from the Danish word “Koagulation”] was isolated in 1930 by the Danish Dam and it is an essential factor required for post-translational modification of coagulation factors II, VII, IX, and X; thus the term Vitamin K is used to indicate some compounds which possess a common 2-methyl-1,4 naftoquinone core, called menadione, and a phytyl chain ide at the 3-position. That side chain is an isoprenoid structure, varying in lengths and degrees of saturation and exhibiting anti-hemorrhagic properties [1].
There are seven molecules which have been designated as vitamin K as shown in Table 1.
Table 1 The seven forms of vitamin K by Fiore et al. [3] (modified). | Vitamin K1 | 2-methyl-3-phythyl-1,4-naphthoquinone | Phylloquinone – phytonadione - phytomenadione |
| Vitamin K2 | 2-methyl-3-difarneasyl-1,4-naphthoquinone | menaquinone –menatetrenone |
| Vitamin K3 | 2-methyl-1,4-naphthoquinone | menadione – menadione Na-bisulfite |
| Vitamin K4 | 2-methyl-naphthalene-1,4-diol | menadiol and its esters (diacetate, dibutyrate) |
| Vitamin K5 | 4-amino-2-methyl-1-naphthol | - |
| Vitamin K6 | 2-methyl-1,4-2-naphthaelendiamine | - |
| Vitamin K7 | 4-amino-3-methyl-1-naphthol | - |
Vitamin K occurs naturally in two forms only, i.e., vitamin K1 and K2, whereas vitamins K3 to K7 are synthetic compounds. Vitamin K1 can be found in plants and vitamin K2 is synthesized by Gram positive, above all anaerobic Bacterioides species, found in normal flora of the large intestine [1]. Vitamin K1 is also known as phylloquinone and vitamin K2 is also designated as menaquinone [1]. The chemical structure of vitamin K1 consists of a basal 2-methyl-1,4-naphthoquinone molecule, indicated as menadione in which the two methyl groups are functionally important, and an isoprenoid side chain containing 20-30 carbon atoms at the C3 position [1]. That side chain may change in length and degree of saturation. Vitamin K1 is the predominant form of vitamin K present in the diet and it is also the most widely used preparation for intravenous administration, although vitamin K1 (phytonadione) is a fat-soluble synthetic derivative identical to the naturally occurring vitamin K1.
Moreover, synthetically prepared vitamins K1, K3, K4, and K5 have been used in clinical practice, and vitamins K3 and K4 are available as water-soluble salts [1]. However, vitamin K1 remains the preferred molecule being the K vitamer available for oral, intramuscular, subcutaneous, and intravenous administration, although oral preparations are not well absorbed [2]. Vitamin K1 is used as an antidote to contrast warfarin overdose [1].
Interestingly, vitamin K1 molecule has two geometrical isomers (cis-trans or (Z)-(E)-isomers) plus two asymmetric carbon atoms (C7 and C11), each generating two enantiomers (R or S). Thus, there are eight diastereoisomers (four in the trans- and four in the cis- configuration). For that reason, in truth, Vitamin K1 is a mixture of 2-methyl-3-[(2E)-(7R,11R)-3,7,11,15-tetramethylhexadec-2-enyl] naphthalene-1,4-dione (trans-phytomenadione), 2-methyl-3-[(2Z)-(7R,11R)-3, 7,11,15-tetra-methylhexadec-2-enyl]naphthalene-1,4-dione [cis-phytomenadione], and 2,3-epoxy-2-methyl-3-[[2E]-[7R,11R]-3,7,11,15-tetramethylhexadec-2-enyl]- 2,3-di hydronaphthalene-1,4-dione (trans-epoxy-phyto-menadione), where, the name Vitamin K1 and its pharmacological properties fits appropriately only for the 2'-Trans-7R, 11R-stereoisomer (the others are not vitamins because the cis-isomer is inactive) [2], and the 2'-Trans-7R, 11R-stereoisomer represents the 75-90% of the mixture, whose chemical formula is shown in Fig. (1).
Fig. (1)) The vitamin K vitamers and the main derivatives.
The liver is the site of synthesis of vitamin K dependent coagulation factors, but the concentration of vitamin K in the hepatic tissue is extremely variable [1]. Furthermore, phylloquinone is a minor component of hepatic vitamin K, being only 10%, whereas menaquinones represent 90% of the liver storage [1] and, although the liver is reputed to be the main catchment area of vitamin K, other tissues as kidney, lung, bone and skin work like reservoire for vitamin K [2]
Vitamin K1 has a molecular weight of 450.68 g/mol and it looks like a clear or golden yellow viscous liquid, insoluble in water, slightly soluble in ethanol, and freely soluble in ether and oils, slowly degraded by oxygen and enough stable to warmth, but strongly light-sensitive. Vitamin K1 in fact, is slowly degraded by atmospheric oxygen, but is relatively rapidly degraded by light [1]. Vitamin K in the organism is oxidized to its inactive metabolite, Vitamin K1 epoxide, i.e., vitamin K1 oxide, as shown in Fig. (1) [1].
Leafy green vegetables like spinach are the main source of vitamin K1, but it is present in broccoli, turnip, soybean, asparagus and carrot juice too [2], and an adequate intake of vitamin K is 90 micrograms for females and 120 micrograms for males daily [1].
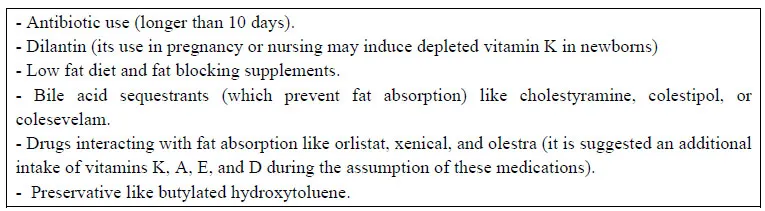
Beyond gastrointestinal and liver diseases, some drugs may interfere with vitamin K absorption as illustrated in Table 2.
Table 2 Medications causing vitamin K deficiency*. *Source: http://umm.edu/health/medical/altmed/supplementinteraction/possible-interactions-with-vitamin-k.
The major use of vitamin K is in the animal farms and it is added to animal feed, above all t...