
eBook - ePub
Pre-Examination Procedures in Laboratory Diagnostics
Walter G. Guder, Walter G. Guder
This is a test
Compartir libro
- 424 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
eBook - ePub
Pre-Examination Procedures in Laboratory Diagnostics
Walter G. Guder, Walter G. Guder
Detalles del libro
Vista previa del libro
Índice
Citas
Preguntas frecuentes
¿Cómo cancelo mi suscripción?
¿Cómo descargo los libros?
Por el momento, todos nuestros libros ePub adaptables a dispositivos móviles se pueden descargar a través de la aplicación. La mayor parte de nuestros PDF también se puede descargar y ya estamos trabajando para que el resto también sea descargable. Obtén más información aquí.
¿En qué se diferencian los planes de precios?
Ambos planes te permiten acceder por completo a la biblioteca y a todas las funciones de Perlego. Las únicas diferencias son el precio y el período de suscripción: con el plan anual ahorrarás en torno a un 30 % en comparación con 12 meses de un plan mensual.
¿Qué es Perlego?
Somos un servicio de suscripción de libros de texto en línea que te permite acceder a toda una biblioteca en línea por menos de lo que cuesta un libro al mes. Con más de un millón de libros sobre más de 1000 categorías, ¡tenemos todo lo que necesitas! Obtén más información aquí.
¿Perlego ofrece la función de texto a voz?
Busca el símbolo de lectura en voz alta en tu próximo libro para ver si puedes escucharlo. La herramienta de lectura en voz alta lee el texto en voz alta por ti, resaltando el texto a medida que se lee. Puedes pausarla, acelerarla y ralentizarla. Obtén más información aquí.
¿Es Pre-Examination Procedures in Laboratory Diagnostics un PDF/ePUB en línea?
Sí, puedes acceder a Pre-Examination Procedures in Laboratory Diagnostics de Walter G. Guder, Walter G. Guder en formato PDF o ePUB, así como a otros libros populares de Médecine y Théorie, pratique et référence de la médecine. Tenemos más de un millón de libros disponibles en nuestro catálogo para que explores.
Información
___
1. General Part
1.1 Introduction and History of the Preanalytical Phase
1.1.1 Introduction
It is extremely important to obtain adequate sample, ensure its appropriate transport and maintain proper storage before medical laboratories can perform diagnostic analysis [1]. Thirtyfive years after the introduction of the term preanalytical phase [2], it is now appropriate to summarize the existing knowledge, terms and recommendations about all biological and technical aspects between the patient and laboratory, which need to be controlled in order to obtain accurate laboratory result.
Most important of the whole diagnostic process, but often not considered as part of the quality criteria, is the extralaboratory part of the total turnaround time (TTT or total TAT) from the medical question to the sample [3]. This has been shown to be the source of a majority of “laboratory errors” [4] and is included in the ISO/ EN/DIN 15189 requirements for quality and competence (ISO 2007). It was only recently that quality indicators of the preanalytical phase were harmonized [5] (see Chapters 8.1–8.3).
Guidelines and diagnostic pathways helped to improve the selection of type of sample, time and site of sampling and also the definition of reference intervals for clinical interpretation of results (see Chapter 1.2). Various biological and technical aspects can either influence the laboratory result in vivo or interfere with the analytical procedure, thus changing the result and causing misinterpretations (see Chapter 1.3). These influences include age and gender differences, circadian rhythms as well as food, herbs and drinking habits (see Chapters 3.1–3.4). In addition, drugs can either change analyte concentrations by their biological actions or interfere with the analytical method used (see Chapter 4.4). Interferences can also becaused by secondary changes in sample color and turbidity by lipaemia, hemolysis or icterus (see Chapters 4.1–4.3). The site of sampling determines the result by biological variables (see Chapters 2.1–2.8). Biological influences can be the cause of differences between serum and plasma (see Chapter 2.6). Besides, anticoagulants, additives or contamination can also cause interferences.
The sampling is performed by varied professionals worldwide. The technical procedure including patient and sample identification, sampling techniques and storage and transport conditions are described in separate chapters (Chapters 5.1–5.3 and Chapter 6.1). An additional list provides information on the present state of knowledge about analyte stability in samples and their temperature dependence as well as stabilizing additives (see 10, Annex).
When a sample reaches the laboratory, several processes are mandatory before the requested analysis is performed (see Chapter 8.2). Individual aspects for the various analytes are discussed separately (on hemostaseology, hematology, acid–base state, biochemical and immunological analytes as well as therapeutic drug monitoring, microbiology and biobanking: see Chapters 7.1–7.7). Auditing and internal and external quality assurance covering all the facets of preanalytical phase are explained in Chapters 8.1–8.3, opening the discussion on unresolved questions [6].
Hopefully, this booklet contributes to the increasing awareness of the multiple aspects of laboratorial diagnostic function. Also, the editors wish to thank all the authors for their competent contributions; they have made this volume an updated component of the ongoing progress in medical treatment.
1.1.2 The long history prior to the appearance of the term preanalytical phase
Table 1.1.1: History of technical products and procedures involved in preanalytical phase
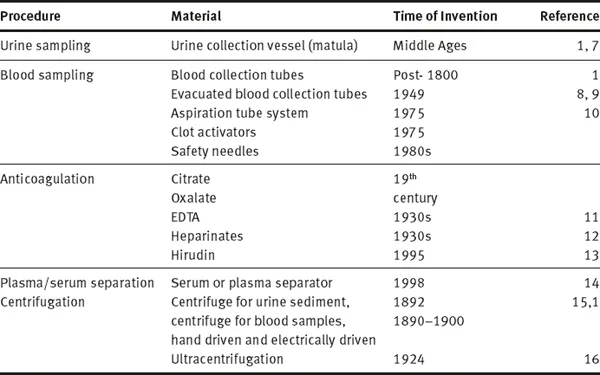
Definition and present state of concepts of preanalytical phase that emerged would not have been possible without more than hundred years of history of the preanalytical phase preceding it. Since chemical and microscopic analysis of body fluids improved medical diagnosis, the type of sample, time of sampling as well as the preparation of patients have been part of the method description. In addition, many inventions and technical improvements contributed to the standards that we use today. Table 1.1.1 summarizes some of the preanalytical techniques and inventions, which although existing since many decenniums are still considered standardized prerequisites for preanalytical procedures. Among the first preanalytical materials documented is the special urine sample container, the “matula” (Fig. 1.1.1), which based on Galen’s humoral theory allowed to separate hypostases (sediment) from sublimia (floating matter) and nubes (cloud).
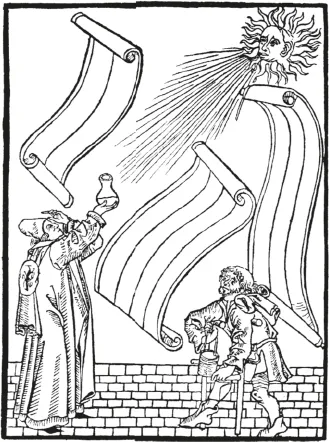
Fig. 1.1.1: Uroscopy showing the preanalytical (man carrying the vessel protecting the matula filled with urine), analytical (uroscopist looking through the urine) and postanalytical phases (hand pointing to the brain of the uroscopist and rolling papers waiting for the prophetic (!) interpretation. (From a wood cut of Steffen Arndes from Lübeck 1510–20; from [17]).
As seen in Fig. 1.1.1 (circa 1500 AD), this matula was carried by the patient to the uroscopist in a matching cylindrical basket, which could be closed with a lid and carried with a handle. The basket protected urine from sunlight that would change the color or turbidity of urine. It was well known that without this protective basket urine was too unstable to get true informations about the color, consistency and contents.
Three hundred years later (post 1800 AD), only when newly developed chemical methods made the detection and quantitation of the constituents of body fluids possible, was blood used for chemical analysis. Initially, whole blood and/or serum, contained in blood tubes standing upright at room temperature for hours, were used as samples. The first use of a centrifuge was for separating urine sediment (Fig. 1.1.2). A hand-driven centrifuge rotor was used to spin two tubes with urine to examine sediment under microscope [15]. This remained a standardized procedure until electrically driven centrifuges with higher speed were available for blood centrifugation from 1920 onwards (although mentioned by Stenbeck in 1892 [15]). Ultimately, the invention of ultracentrifuge facilitated the separation of molecules according to molecular weight and specific weight [16]. Anticoagulants to preserve samples were used in the second half of the 19th century (citrate and oxalate, later EDTA and heparinates and recently hirudin), while standardized EDTA plasma was only possible after the 1940s [11]. These anticoagulants were added to a vessel before letting the blood flow into it and could only be roughly quantitated. Only with the invention of vacuum tubes and aspiration tubes [9, 10] standard volumes and anticoagulant concentrations were achieved. Years later, serum or plasma separators and additives stimulating coagulation in plastic tubes were introduced.

Fig. 1.1.2: Hand driven centrifuge used to form urine sediment.
It is well known among clinical scientists that preparation of the patient for sampling (diet, prolonged fasting prior to sampling, posture before and during sampling, physical activity, etc.), time and site of sampling (early morning versus afternoon, venous or capillary sample, etc.), choice of anticoagulant (serum or citrated-, EDTA- or heparinized blood, etc.), transport and storage (whole blood or serum, room temperature or cold, etc.) and centrifugation time and temperature exert their influence on laboratory results [18, 19]. However, the influences of these factors were not quantitated. Because their impact ...