![]()
Bandello F, Battaglia Parodi M (eds): Anti-VEGF.
Dev Ophthalmol. Basel, Karger, 2010, vol 46, pp 4-20
______________________
Mechanisms of Ocular Angiogenesis and Its Molecular Mediators
Martin J. Siemerinka · Albert J. Augustinb · Reinier O. Schlingemanna,c
aOcular Angiogenesis Group, Department of Ophthalmology, Academic Medical Center, Amsterdam, The Netherlands; bAugenklinik, Karlsruhe, Germany, and cNetherlands Institute for Neuroscience, Royal Netherlands Academy of Arts and Sciences, Amsterdam, The Netherlands
______________________
Abstract
Angiogenesis is defined as the formation of new blood vessels from the existing vasculature. It is a highly coordinated process occurring during development of the retinal vasculature, ocular wound healing, and in pathological conditions. Complex interactions are involved between non-vascular and microvascular cells, such as endothelial cells and pericytes, via several angiogenic growth factors and inhibitors. Of these growth factors, vascular endothelial growth factor (VEGF) has emerged as the single most important causal agent of angiogenesis in health and disease in the eye. During the angiogenic process, endothelial cells shift from a homogeneous quiescent population into a population of heterogeneous phenotypes, each with a distinct cellular fate. So far, three angiogenic specialized phenotypes have been identified: (1) ‘tip cells’, which pick up guidance signals and migrate through the extracellular matrix; (2) ‘stalk cells’, which proliferate, form junctions, produce extracellular matrix, and form a lumen, and (3)‘phalanx cells’, which do not proliferate, but align and form a smooth monolayer. Eventually, a robust mature new blood vessel is formed which is capable of supplying blood and oxygen to tissues. Pathological angiogenesis is a key component of several irreversible causes of blindness. In most of these conditions, angiogenesis is part of a wound healing response culminating, via an angiofibrotic switch, in fibrosis and scar formation which leads to blindness. Currently, VEGF-A antagonists are standard care in the treatment of exudative age-related macular degeneration, and have been found to be a valuable additional treatment strategy in several other vascular retinal diseases.
Copyright © 2010 S. Karger AG, Basel
Blood vessels form an intricate hollow network of arteries, capillaries, and veins for the transport of liquids, solutes, gases, macromolecules, and cells throughout the vertebrate body. The vascular network is formed during early stages of development, and its correct and early function is absolutely critical for survival of the embryo. New blood vessels originate from endothelial precursor cells (angioblasts) by a process called vasculogenesis or from preexisting blood vessels by angiogenesis [1, 2]. Once a functional adult vascular system has been formed completely, blood vessels become quiescent. The growth potential of smaller blood vessels, however, is retained and is employed during wound healing and tissue regeneration.
Beyond its physiological roles, angiogenesis is also a hallmark of many pathological conditions, including neovascular diseases in the eye [3-5]. Excessive angiogenesis occurs when diseased cells produce abnormal amounts of angiogenic factors, overwhelming the effects of natural angiogenesis inhibitors. As the newly formed vessels mainly serve a role in a wound healing response, they usually do not restore the tissue integrity, but rather cause visual impairment when they are located in normally avascular, transparent tissues such as the cornea and vitreous. Strategies for inhibition of angiogenesis include approaches that can block the angiogenesis cascade at several steps [4, 6].
Angiogenesis: Mechanisms and Molecular Mediators
Endothelial Cell Differentiation
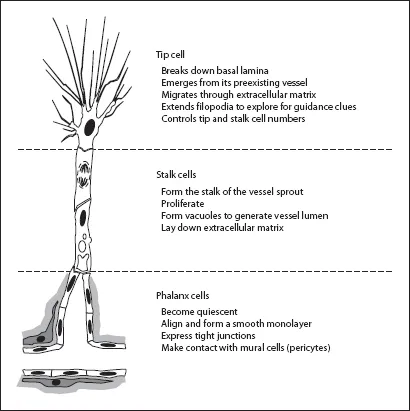
All blood vessels are lined by endothelial cells (ECs), which form the interface between circulating blood in the lumen and the rest of the vessel wall. Under normal conditions, ECs are a remarkably quiescent cell type, undergoing division approximately once every 1,000 days, but when activated, cell division can occur every 1-2 days [7]. Sprouting angiogenesis requires selection of ECs from an existing blood vessel which will be activated to form the new vessel, while at the same time, surrounding ECs remain quiescent in their current position. From recent studies a model has emerged in which ECs differentiate into three specialized cell types with distinct phenotypes during angiogenesis (fig. 1) [8-10]. First, a single ‘tip cell’ develops. This EC breaks down the basal lamina, emerges from its parent blood vessel and becomes the leading cell of the sprouting vessel. The tip cell migrates into the extracellular matrix and senses microenvironmental attractive and repulsive signals for guidance. Secondly, following directly behind the migrating tip cell, other ECs differentiate under the influence of the adjacent tip cell into ‘stalk cells’ that proliferate and bridge the gap between the tip cell and the parent vasculature. Stalk cells generate the blood vessel lumen through the formation of intracellular vacuoles, a process called ‘lumenogenesis’. Thirdly, ECs behind the stalk cells differentiate into ‘phalanx cells’, and align in a smooth cobblestone monolayer, becoming the most inner cell layer in the new blood vessel. Phalanx cells no longer proliferate, express tight junctions and make contact with mural cells.
Angiogenesis Inducers and Inhibitors
Angiogenesis is tightly controlled by closely interacting angiogenic and angiostatic factors, and their balance ultimately determines if, where and when the ‘angiogenic switch’ is turned on with angiogenesis as the result [2, 9]. Over the past decades, numerous inducers of angiogenesis have been identified, including the members of the vascular endothelial growth factor (VEGF) family, angiopoietins, transforming growth factors (TGF), epidermal growth factor (EGF), platelet-derived growth factor (PDGF), tumor necrosis factor-α (TNF-α), insulin-like growth factor (IGF), vascular endothelial-cadherin (VE-cadherin), interleukins and the members of the fibroblast growth factor (FGF) family. In addition, there is a plethora of growth factors, hormones and metabolites that have been reported to directly or indirectly stimulate physiological and pathological angiogenesis (table 1) [11, 12]. Not all of these factors are specific for ECs. Consistent with a major role for hypoxia in the overall process of angiogenesis, a large number of angiogenic factors involved in various stages of angiogenesis are independently responsive to hypoxia [13]. The VEGF family of proteins is the most important family of angiogenic factors that controls blood vessel formation.
Fig. 1. Representative model of sprouting angiogenesis. At least three different angiogenic specialized endothelial cells (white) are required, each with ...