
eBook - ePub
The HPLC-MS Handbook for Practitioners
Stavros Kromidas, Stavros Kromidas
This is a test
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
eBook - ePub
The HPLC-MS Handbook for Practitioners
Stavros Kromidas, Stavros Kromidas
Detalles del libro
Vista previa del libro
Índice
Citas
Información del libro
Filling the gap for an expert text dealing exclusively with the practical aspects of HPLC-MS coupling, this concise, compact, and clear book provides detailed information to enable users to employ the method most efficiently.
Following an overview of the current state of HPLC-MS and its instrumentation, the text goes on to discuss all relevant aspects of method development. A chapter on tips and tricks is followed by user reports on the advantages - and pitfalls - of applying the method in real-life scenarios. The whole is rounded off by a look at future developments by renowned manufacturers.
Preguntas frecuentes
¿Cómo cancelo mi suscripción?
¿Cómo descargo los libros?
Por el momento, todos nuestros libros ePub adaptables a dispositivos móviles se pueden descargar a través de la aplicación. La mayor parte de nuestros PDF también se puede descargar y ya estamos trabajando para que el resto también sea descargable. Obtén más información aquí.
¿En qué se diferencian los planes de precios?
Ambos planes te permiten acceder por completo a la biblioteca y a todas las funciones de Perlego. Las únicas diferencias son el precio y el período de suscripción: con el plan anual ahorrarás en torno a un 30 % en comparación con 12 meses de un plan mensual.
¿Qué es Perlego?
Somos un servicio de suscripción de libros de texto en línea que te permite acceder a toda una biblioteca en línea por menos de lo que cuesta un libro al mes. Con más de un millón de libros sobre más de 1000 categorías, ¡tenemos todo lo que necesitas! Obtén más información aquí.
¿Perlego ofrece la función de texto a voz?
Busca el símbolo de lectura en voz alta en tu próximo libro para ver si puedes escucharlo. La herramienta de lectura en voz alta lee el texto en voz alta por ti, resaltando el texto a medida que se lee. Puedes pausarla, acelerarla y ralentizarla. Obtén más información aquí.
¿Es The HPLC-MS Handbook for Practitioners un PDF/ePUB en línea?
Sí, puedes acceder a The HPLC-MS Handbook for Practitioners de Stavros Kromidas, Stavros Kromidas en formato PDF o ePUB, así como a otros libros populares de Physical Sciences y Analytic Chemistry. Tenemos más de un millón de libros disponibles en nuestro catálogo para que explores.
Información
Part I
Overview, Pitfalls, Hardware-Requirements
1
State of the Art in the LC/MS
O. Schmitz
1.1 Introduction
The dramatically increased demands on the qualitative and quantitative analysis of more complex samples are a huge challenge for modern instrumental analysis. For complex organic samples (e.g., body fluids, natural products or environmental samples), only chromatographic or electrophoretic separations followed by mass spectrometric detection meet these requirements. However, at the moment a tendency can be observed, in which a complex sample preparation and preseparation is replaced by high-resolution mass spectrometer with atmospheric pressure ion sources. However, numerous ion–molecule reactions in the ion source – especially in complex samples due to incomplete separation – are possible because the ionization in typical atmospheric pressure ion sources is nonspecific [1]. Thus, this approach often leads to ion suppression and artifact formation in the ion source, particularly in electrospray ionization (ESI) [2].
Nevertheless, sources such as ASAP (atmospheric pressure solids analysis probe), DART (direct analysis in real time), and DESI (desorption electrospray ionization) can often be successfully used. In ASAP, a hot nitrogen flow from an ESI or APCI (atmospheric pressure chemical ionization) source is used as a source of energy for evaporation and the only change to an APCI source is the installation of an insertion option to place the sample in the hot gas stream within the ion source [3]. This ion source allows a rapid analysis of volatile and semivolatile compounds and, for example, was used to analyze biological tissue [3], polymer additives [3], fungi and cells [4], and steroids, [3, 5]. ASAP has much in common with DART [6] and DESI [7]. The DART ion source produces a gas stream containing long-lived electronically excited atoms that can interact with the sample and, thus, desorption and subsequent ionization of the sample by Penning ionization [8] or proton transfer from protonated water clusters [6] is realized. The DART source is used for the direct analysis of solid and liquid samples. A great advantage of this source is the possibility to analyze compounds on surfaces such as illegal substances on dollar bills or fungicides on wheat [9]. Unlike ASAP and DART, the great advantage of DESI is that the volatility of the analyte is not a prerequisite for a successful analysis (same as in the classic ESI). DESI is most sensitive for polar and basic compounds and less sensitive for analytes with a low polarity [10]. These useful ion sources have a common drawback. All or almost all substances in the sample are present at the same time in the gas phase during the ionization in the ion source. The analysis of complex samples can therefore lead to ion suppression and artifact formation in the atmospheric pressure ion source due to ion–molecule reactions on the way to the MS inlet. For this reason, some ASAP applications are described in the literature with increasing temperature of the nitrogen gas [5, 11, 12]. DART analyzes with different helium temperatures [13] or with a helium temperature gradient [14] have been described in order to achieve a partial separation of the sample due to the different vapor pressures of the analyte. Related with DART and ASAP, the direct inlet sample APCI (DIP-APCI) from Scientific Instruments Manufacturer GmbH (SIM) was described 2012, which uses a temperature-push rod for direct intake of solid and liquid samples with subsequent chemical ionization at atmospheric pressure [15]. Figure 1.1 shows a DIP-APCI analysis of a saffron sample (solid, spice) without sample preparation with the saffron-specific biomarkers isophorone and safranal. As a detector, an Agilent Technologies 6538 UHD Accurate-Mass Q-TOF was used. The total ion chromatogramm (TIC) of the total analysis and the mass spectrum at the time of 2.7 min are shown in Figure 1.1a,b, respectively. The analysis was started at 40 °C and heated the sample at 1 K/s to a final temperature of 400 °C.
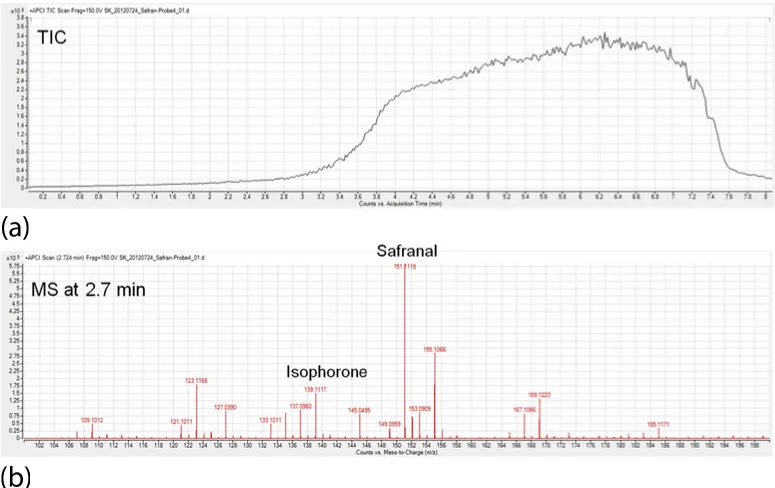
Figure 1.1 Analysis of saffron using direct-inlet probe-APCI with high-resolution QTOF-MS. (a) TIC of the toal analysis. (b) mass spectrum at the time of 2.7 min.
These ion sources may be useful and time saving but for the quantitative and qualitative analysis of complex samples a chromatographic or electrophoretic preseparation makes sense. In addition to the reduction of matrix effects, the comparison of the retention times also allows an analysis of isomers.
1.2 Ionization Methods at Atmospheric Pressure
In the last 10 years, several new ionization methods for atmospheric pressure (AP) mass spectrometers have been developed. Some of these are only available in some working groups. Therefore, only four commercially available ion sources will be presented in detail here.
The most common atmospheric pressure ionization (API) is electrospray ionisation (ESI), followed by APCI and APPI (atmospheric pressure photoionization). A significantly lower significance shows the APLI (atmospheric pressure laser ionization). However, this ion source is well suited for the analysis of aromatic compounds and, for example, the gold standard for PAH (polyaromatic hydrocarbons) analysis. This ranking reflects more or less the chemical properties of the analytes, which are determined with API MS: Most analytes from the pharmaceutical and life sciences are polar or even ionic and, thus, are efficiently ionized by ESI (Figure 1.2). However, there is also a considerable interest in API techniques for efficient ionization of less or nonpolar compounds. For the ionization of such substances ESI is less suitable.
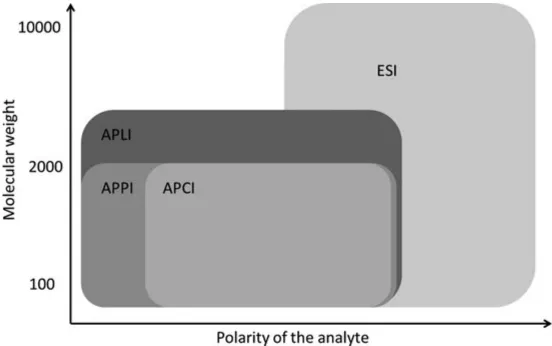
Figure 1.2 Polarity range of analytes for ionization with various atmospheric pressure ionization (API) techniques. Note: The extended mass range of APLI against APPI and APCI results from the ionization of nonpolar aromatic analytes in an electrospray Reproduced with kind permission of O. J. Schmitz, T. Benter, Advances in LC-MS Instrumentation: Atmospheric pressure laser ionization, Journal of Chomatography Libary, Vol 72 (2007), Chapter 6, Pages 89-113.
1.2.1 Overview of API Methods
Ionization methods that operate at atmospheric pressure, such as atmospheric pressure chemical ionization (APCI) and electrospray ionization (ESI), have greatly expanded the scope of mass spectrometry [17–20]. These API techniques allow an easy coupling of chromatographic separation systems, such as liquid chromatography (LC), to a mass spectrometer.
There is a fundamental difference between APCI and ESI ionization mechanism. In APCI, ionization of the analyte takes place in the gas phase after evaporation of the solvent. In ESI, the ionization takes place already in the liquid phase. In the ESI process, protonated or deprotonated molecular ions are usually formed from highly polar analytes. Fragmentation is rarely observed. However, for the ionization of less polar substances, APCI is preferably used. APCI is based on the reaction of analytes with primary ions, which are generated by corona discharge. But the ionization of nonpolar analytes is very low with both techniques.
For these classes of substances other methods have been developed, such as the coupling of ESI with an electrochemical cell [21–32], the “coordination ionspray” [32–47] or the “diss...
Índice
Estilos de citas para The HPLC-MS Handbook for Practitioners
APA 6 Citation
Kromidas, S. (2017). The HPLC-MS Handbook for Practitioners (1st ed.). Wiley. Retrieved from https://www.perlego.com/book/992656/the-hplcms-handbook-for-practitioners-pdf (Original work published 2017)
Chicago Citation
Kromidas, Stavros. (2017) 2017. The HPLC-MS Handbook for Practitioners. 1st ed. Wiley. https://www.perlego.com/book/992656/the-hplcms-handbook-for-practitioners-pdf.
Harvard Citation
Kromidas, S. (2017) The HPLC-MS Handbook for Practitioners. 1st edn. Wiley. Available at: https://www.perlego.com/book/992656/the-hplcms-handbook-for-practitioners-pdf (Accessed: 14 October 2022).
MLA 7 Citation
Kromidas, Stavros. The HPLC-MS Handbook for Practitioners. 1st ed. Wiley, 2017. Web. 14 Oct. 2022.