
eBook - ePub
Molecular Genetics of Bacteria
Jeremy W. Dale, Simon F. Park
This is a test
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Molecular Genetics of Bacteria
Jeremy W. Dale, Simon F. Park
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
The fifth edition of this highly successful book provides students with an essential introduction to the molecular genetics of bacteria covering the basic concepts and the latest developments. It is comprehensive, easy to use and well structured with clear two-colour diagrams throughout.
Specific changes to thenew edition include:
- More detail on sigma factors, anti-sigma factors and anti-anti sigma factors, and the difference in the frequency of sigma factors in bacteria
- Expand material on integrons as these are becoming increasingly important in antibiotic resistance
- Enhanced treatment of molecular phylogeny
- Complete revision and updating of the final chapter on 'Gene Mapping and Genomics'
- Two-colour illustrations throughout.
The focus of the book remains firmly on bacteria and will be invaluable to students studying microbiology, biotechnology, molecular biology, biochemistry, genetics and related biomedical sciences.
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Molecular Genetics of Bacteria est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Molecular Genetics of Bacteria par Jeremy W. Dale, Simon F. Park en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Biological Sciences et Microbiology. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
1
Nucleic Acid Structure and Function
In this book it is assumed that you will already have a working knowledge of the essentials of molecular biology, especially the structure and synthesis of nucleic acids and proteins. The purpose of this chapter therefore is to serve as a reminder of some of the most relevant points, and to highlight those features that are particularly essential for an understanding of later chapters.
1.1 Structure of nucleic acids
1.1.1 DNA
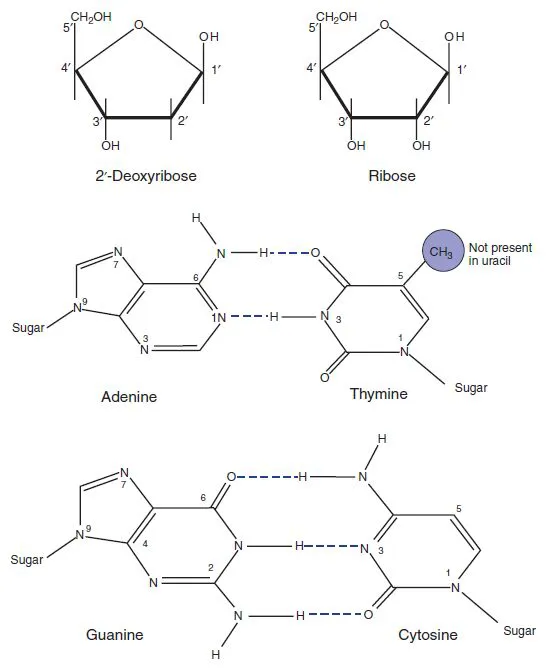
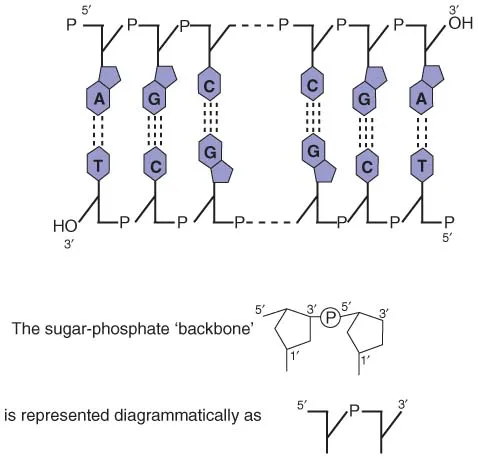
In bacteria, the genetic material is double-stranded DNA, although bacteriophages (viruses that infect bacteria; see Chapter 4) may have double-stranded or single-stranded DNA, or RNA. The components of DNA (Figure 1.1) are 2′-deoxyribose (forming a backbone in which they are linked by phosphate residues) and four heterocyclic bases: two purines (adenine, A, and guanine, G) and two pyrimidines (thymine, T, and cytosine, C). The sugar residues are linked by phosphodiester bonds between the 5′ position of one deoxyribose and the 3′ position of the next (Figure 1.2), while one of the four bases is attached to the 1′ position of each deoxyribose. It is the sequence of these four bases that carries the genetic information.
The two strands are twisted around each other in the now familiar double helix, with the bases in the centre and the sugar-phosphate backbone on the outside. The two strands are linked by hydrogen bonds between the bases. The only arrangement of these bases that is consistent with maintaining the helix in its correct conformation is when adenine is paired with thymine and guanine with cytosine. One strand therefore consists of an image of the other; the two strands are said to be complementary. Note that the purines are larger than the pyrimidines, and that this arrangement involves one purine opposite a pyrimidine at each position, so the distance separating the strands remains constant.
Figure 1.1 Structure of the basic elements of DNA and RNA. RNA contains ribose rather than deoxyribose, and uracil instead of thymine.
1.1.2 RNA
The structure of RNA differs from that of DNA in that it contains the sugar ribose instead of deoxyribose, and uracil instead of thymine (Figure 1.1). It is usually described as single-stranded, but only because the complementary strand is not normally made. There is nothing inherent in the structure of RNA that prevents it forming a double-stranded structure: an RNA strand will pair with (hybridize to) a complementary RNA strand, or with a complementary strand of DNA. Even a single strand of RNA will fold back on itself to form double-stranded regions. In particular, transfer RNA (tRNA), and ribosomal RNA (rRNA) both form complex patterns of base-paired regions. The formation of secondary and tertiary structures in RNA via base-pairing can also influence gene expression and this is considered in further detail in Chapter 3.
Figure 1.2 Diagrammatic structure of DNA.
1.1.3 Hydrophobic interactions
Although geneticists emphasize the importance of the hydrogen bonding between the two DNA strands, these are not the only forces influencing the structure of the DNA. The bases themselves are hydrophobic, and will tend to form structures in which they are removed from the aqueous environment. This is partially achieved by stacking the bases on top of one another (Figure 1.3). The double-stranded structure is stabilized by additional hydrophobic interactions between the bases on the two strands. The hydrogen bonding not only holds the two strands together but also allows the corresponding bases to approach sufficiently closely for the hydrophobic forces to operate. The hydrogen bonding of the bases is, however, of special importance because it gives rise to the specificity of the base-pairing between the two chains.
Figure 1.3 Hydrophobic interactions of bases in DNA. The hydrophobic bases stack in the centre of the helix, reducing their contact with water.
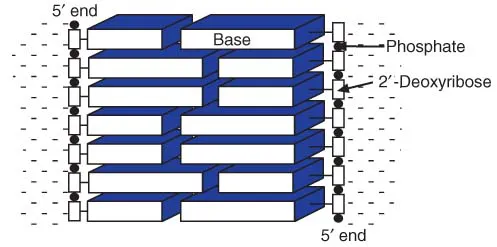
Although the bases are hydrophobic, and therefore very poorly soluble in water, nucleic acids are quite soluble, due largely to the hydrophilic nature of the backbone, and especially the high concentration of negatively charged phosphate groups. This will also tend to favour a double-helical structure, in which the hydrophobic bases are in the centre, shielded from the water, and the hydrophilic phosphate groups are exposed.
1.1.4 Different forms of the double helix
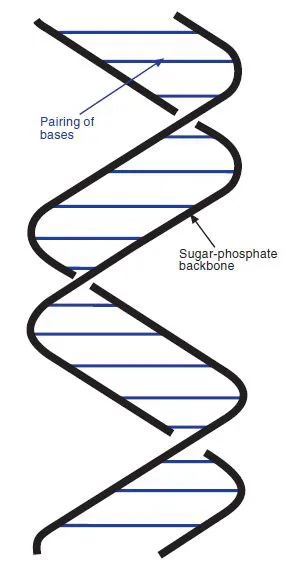
A full consideration of DNA structure would be extremely complex, and would have to take into account interactions with the surrounding water itself, as well as the influence of other solutes or solvents. The structure of DNA can therefore vary to some extent according to the conditions. In vitro, two main forms are found. The Watson and Crick structure refers to the B form, which is a right-handed helix with 10 base-pairs per turn (Figure 1.4). Under certain conditions, isolated DNA can adopt an alternative form known as the A form, which is also a right-handed helix, but more compact, with about 11 base pairs (bp) per turn. Within the cell, DNA resembles the B form more closely, but has about 10.4 bp per turn (it is underwound; see below).
Certain DNA sequences, notably those containing alternating G and C residues, tend to form a left-handed helix, known as the Z form (since the sugar-phosphate backbone has a zigzag structure rather than the regular curve shown in the B form). Although Z DNA was originally demonstrated using synthetic oligonucleotides, naturally occurring DNA within the cell can adopt a left-handed structure, at least over a short distance or temporarily. The switch from left- to right-handed can have important influences on the expression of genes in that region.
Figure 1.4 Diagrammatic structure of B-form DNA. The two anti-parallel sugarphosphate chains form a right-handed helix, with the bases in the centre, held together by hydrophobic interactions and hydrogen bonding.
1.1.5 Supercoiling
Within the cell, the DNA helix is wound up into coils; this is known as supercoiling. Figure 1.5. shows a simple demonstration of supercoiling, which you can easily try out for yourself. Take a strip of paper, and twist one end to introduce one complete turn (i.e. the same side of the paper is facing you at each end). It will now look as in Figure 1.5a. Then bring the two ends towards each other; the conformation will change to that shown in Figure 1.5b, which is a simple form of supercoiling. Notice that not only has the strip of paper become supercoiled, but also the degree of twisting seems to have changed (in this example it now appears not to be twisted at all). If you have kept hold of both ends, the twist of the strip cannot have disappeared completely; it has merely changed to a different form. If you pull the ends apart again, it will change back to the form shown in Figure 1.5a.
Figure 1.5 Interaction between twisting and supercoiling. (a) A ribbon with a single complete twist, without supercoiling. (b) The same ribbon, allowed to form a supercoil; the ribbon is now not twisted.
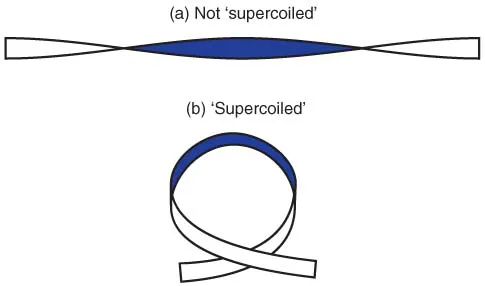
There are three parameters involved: twist (T), linking number (L) and writhe (W). The twist is the number of turns of the strip whereas writhe (essentially a measure of the degree of supercoiling) can be considered as the number of times the strip crosses over itself in a defined direction. These two parameters vary according to the conformation: in Figure 1.5a there is one twist (T = 1) but no supercoiling (W = 0), whereas in Figure 1.5b there is no twist (T = 0) and the strip crosses itself once (W = 1). The linking number, which is a measure of the overall twisting of the strip, is equal to the sum of the other two parameters, i.e. L = T + W.
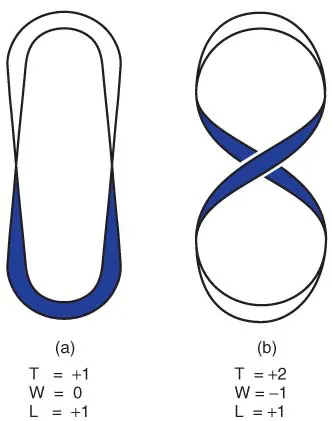
If the ends of the strip are not free to rotate, then the linking number will remain constant. Most of the DNA molecules we will be considering are circular, and therefore do not contain ends that can rotate. Unless there is a break in the DNA, any change in the twist will be balanced by a change in supercoiling, and vice versa. This is illustrated by Figure 1.6.
In Figure 1.6a the strip (or DNA molecule) is not supercoiled (W = 0) but contains one complete twist (T = +1); the linking number (L) is +1. In Figure 1.6b the overall shape has been changed by rotating one end of the structure (i.e. introducing a degree of supercoiling). The strip crosses itself once, and by convention a crossover in this direction is assigned a negative value, so W = −1. At the same time the twist has changed; there are now two complete twists, so T = +2. Since L = T + W, we see that L remains the same (+1).
Figure 1.6 Supercoiling of a circular molecule. In (a), the ‘molecule’ has one twist and no supercoils. Rotating one end of the molecule (b) introduces negative supercoils and increases the amount of twisting. Since the linking number (L) remains the same, the two forms are interchangeable without breaking the circle. See the text for further explanation.
The two structures shown in Figure 1.6 are interchangeable by rotating one end, without opening the circle. With an intact circle, you can change the twist and the writhe jointly but not separately. Any change in supercoiling will involve a compensating change in the twist (and vice versa) so that the linking number remains constant. It is only possible to alter the linking number in circular DNA by breaking and rejoining DNA strands, for example through the action of topoisomerases (see below).
Bacterial DNA is normally negatively supercoiled. Another way of putting it is to say that the DNA is underwound so that if the DNA was not supercoiled, the degree of twisting of the helix would be less than that seen in relaxed linear DNA. If the DNA is nicked (i.e. one strand is broken, leaving it free to rotate) it relaxes into an open circular, non-supercoiled form. Chromosomal DNA is usually broken into linear fragments during lysis of the cell, but bacterial plasmids (see Chapter 5) are usually small enough to be isolated intact in a supercoiled form.
The compact supercoiled structure of the DNA is also significant in that the chromosome, in its expanded state, would be a thousand times longer (about 1 mm) than the bacterial cell itself. To put it another way, a bacterial operon of four genes, in its non-supercoiled B form, would stretch from one end of the cell to the other. Supercoiling is only the start of story, as the bacterial chromosome consists of a large number of supercoiled loops arranged on a core to produce a highly compact and organized structure known as the nucleoid. Supercoiling (and other structural features) of the DNA are also important in the regulation of gene expression (see Chapter 3).
Action of topoisomerases
Supercoiling of bacterial DNA is not achieved by physically twisting the circular molecule in the way we illustrated in Figure 1.6. Instead the cell uses enzymes known as DNA topoisomerases to introduce (or remove) supercoils from DNA by controlled breaking and rejoining of DNA strands.
DNA topoisomerases can be considered in two classes. Type I topoisomerases act on a segment of DNA by breaking one of the strands and passing the other strand through the gap, followed by resealing the nick. Since this increases the number of times the two strands cross one another, the linking number is increased by 1, which results in an increase in either T or W. The Escherichia coli topoisomerase I acts only on negatively supercoiled DNA; the increase in the value of W means that the degree of negative supercoiling is reduced (the DNA becomes relaxed).
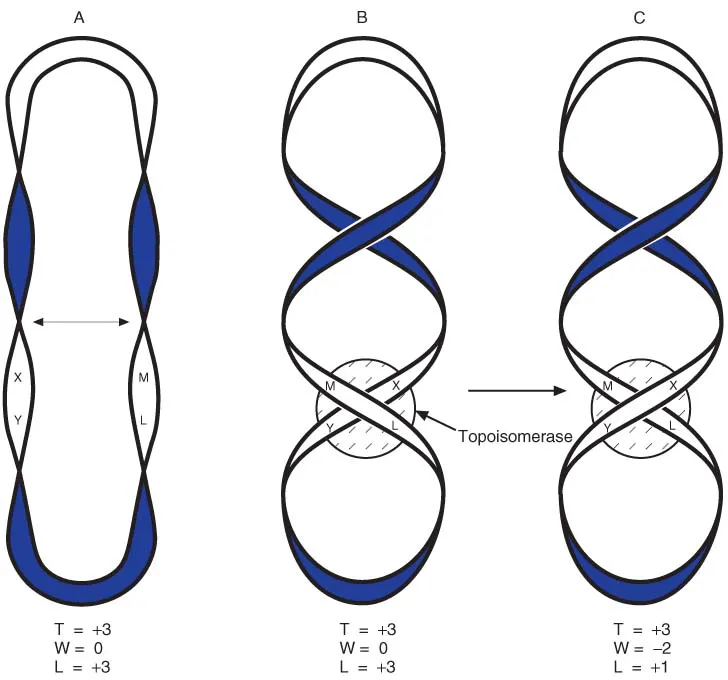
Type II topoisomerases break both strands and pass another duplex region through the gap. In Figure 1.7, we start with a non-supercoiled circle (structure A) and move the centres of the right- and left-hand loops across one another to give structure B. Although this may look at first glance to be supercoiled, it is not. The two crossovers are in opposite directions and therefore cancel one another out. (Mathematically W = −1 at the upper point, and W = +1 at the lower one, so overall W = 0.) If both strands of the helix are broken between points L and M, and the lower strands (X − Y) are moved through the gap, followed by resealing the strands between L and M, structure C is formed. Now, both crossovers are in the same direction, so the structure is supercoiled (W = −2). There is a corresponding reduction in the linking number. The enzyme has introduced a negative supercoil, and at the same time has reduced the winding of the helix. An important example of this type of enzyme is DNA gyrase, which is able to introduce negative supercoils into newly replicated DNA.
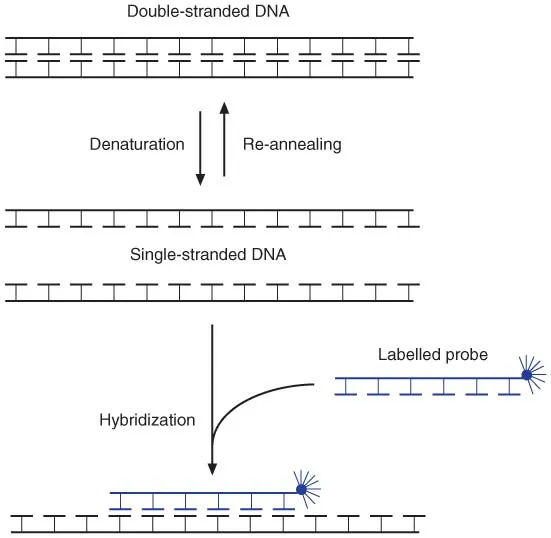
1.1.6 Denaturation and hybridization
Since the two strands of DNA are only linked by non-covalent forces, they can easily be separated in the laboratory, for example by increased temperature or high pH. Separation of the two DNA strands, denaturation, is readily reversible. Reducing the temperature, or the pH, will allow hydrogen bonds between complementary DNA sequences to reform; this is referred to as re-annealing (Figure 1.8). If DNA molecules from different sources are denatured, mixed and allowed to re-anneal, it is possible to form hydrogen bonds between similar DNA sequences (hybridization). This forms the basis of the use of DNA probes to detect specific DNA sequences. The specificity can be adjusted by altering the conditions used for re-annealing (or subsequent washing). Higher temperature, or lower ionic strength, gives greater stringency of hybridization. Highstringency hybridization is used to detect closely related sequences, or to distinguish between sequences with only small differences, whereas lowstringency conditions are used to detect sequences that are more remotely related to your probe. This technique forms an important part of modern molecular biology, and we will encounter many applications in subsequent chapters.
Figure 1.7 Action of Type II topoisomerase. Structure A is not supercoiled, and is converted to B by bending the two sides as shown by the arrow. B is not supercoiled: the two crossing points are of opposite sign and cancel one another. The topoisomerase makes a double-strand break between L and M, passes the X-Y region through the gap, and re-seals the break between L and M. This changes the sign of W at that point, so structure C is now negatively supercoiled.
Temporary separation of localized regions of the two DNA strands also occurs as an essential part of the processes of replication and transcription.
Figure 1.8 Denaturation and hybridization of DNA.
Note that there are three hydrogen bonds linking guanine and cytosine while the adenine–thymine pairing has only two hydrogen bonds. The two DNA strands are therefore more strongly attached in those regions with a high G + C content. Because of this, such regions are more resistant to denaturation and...