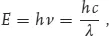![]()
Chapter 1
A Scenic Route through the Laser
1.1 The Meaning of “Laser”
“Laser” is one of the rare acronyms whose meaning has not been lost over five decades. It stands for Light Amplification by Stimulated Emission Radiation. These words are not sufficient to clarify the meaning, unless we have a picture associated to each of them in our mind. The next few sections will be devoted to unraveling the meaning of each term.
1.1.1 Light (Photon) is a Wave
The first letter “L” tells us that “laser” is “light”. Light is an old known entity originating from the sun and the moon. Once it was associated with fire and thought to be the first essential element. In modern language we say that light actually consists of photons, just as matter is made of atoms. Our intuitive picture of atoms is that they can be nicely classified by their mass in a table – the Mendeleev table. Atoms themselves are boxes filled with electrons, protons and neutrons, and there is a mass associated with each component.
Atoms can combine to make molecules, the ultimate component we expect to arrive at when grinding to its finest constituent any piece of material, from a live leaf to a piece of paper. In a way molecules and atoms are what we deal with on a daily basis, but at such a fine scale that it escapes our direct perception. Photons are as ubiquitous, but quite different from atoms and their constituents. Ubiquitous, because they are associated not only with visible light, but also with invisible radiation (infrared and ultraviolet), x-rays, gamma rays, radio waves, and even the radiation from our electrical network at 50 or 60 Hz. They are quite different because there is no mass associated to the photon. A wave is associated with the photon, which is an oscillation propagating at the speed of light.
What is a wave? There is always a pattern and motion associated to the wave; the ripples of a stone thrown in a pond or folds of a flag. One can imagine more and more examples that the word “wave” is applied to. As physicists we would like to pause and clarify some features of the wave with definitions that can be used to quantify similar observations. If you take a picture from the ripples on the pond you realize that there are regular patterns that repeat in water, and you can possibly count the number of peaks on the water surface, that are separated by a “wavelength”. You can also only consider a fixed point on the surface and monitor its motion as it goes up and down, or oscillates. It takes a “period” for each point on the pond to repeat its position. The pattern on the wave (for example, the peaks) have a certain “speed” or “wave velocity”, and the peaks that are created by the wave have an “amplitude”. It is reasonable to conclude that stronger waves have bigger amplitudes, but there is more to the strength or energy of a wave, as we will see in the following sections.
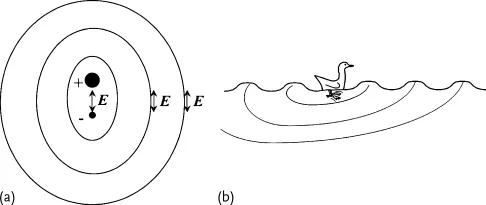
When a wave goes through a medium it does not mean that the medium is necessarily moving with it. In the case of a flag waving in the wind, there is a wave that goes through the flag, but the fabric itself is not carried away. The wave propagates for huge distances, while each particle responsible for the wave motion stays at the same average position, just inducing the motion of the next particle. In most cases, the wave starts from a local oscillation (Figure 1.1a), and propagates radially from there, like rings produced by a duck paddling on a pond (Figure 1.1b). In the case of light, it is the electric field produced by a charge oscillating up and down that starts off the wave. This is called dipole emission.
The velocity of a wave is a property of the medium in which the wave propagates. Sound waves propagate at 343 m/s (1125 ft/s) in dry air at room temperature and faster in denser media. The opposite holds for light waves that usually travel faster in air or vacuum. There are different types of waves. Mechanical waves like spring oscillations and sound waves are due to mechanical motion of particles. The oscillation of these waves is along the propagation direction. Light waves, however, are electromagnetic waves, which originate from the oscillation of charges (electrons, for example). This was the first dilemma in early attempts to interpret light waves: what is moving?
Our intuition is shaped by the observation of water waves in a pond, an oscillating spring, or the swing of a pendulum. These are all mechanical waves. Like sound waves, they require a medium; they need matter to exist. Hence was born the notion of the “ether”, a (fictitious?) medium to support the propagation of light waves. Today the “ether” has simply given way to vacuum, but it does not mean that the understanding of the nature of light has become simpler.
As will be explained in Section 1.1.2 below, quantum mechanics tells us that the amplitude of the positive–negative charge oscillation is restricted to discrete values. Consequently, the emitted oscillation also takes discrete values, to which is associated an energy: the photon energy hν, where ν the frequency of the oscillation, and h is called the “Planck constant” (see Eq. (1.1) in the next section). It is as if the duckling in Figure 1.1a had discrete gears to activate his webbed paws. What is more puzzling is that the “neutral” gear is missing. The minimum energy state of the quantum harmonic oscillator is not zero, but (1/2)hν. This is often referred to as vacuum fluctuation or zero point energy. The absence of vacuum (the ether concept) has been replaced by an absence of zero energy. Since, according to Einstein, there is an equivalence of matter and energy, the two concepts are not so far apart.
1.1.2 Photon Energy
Quantum mechanics tells us that a photon has dual characteristics, it acts both as a wave (Section 1.1.1) and as a particle. In a way, the photon is a wave that can be counted. This might be a bit hard to digest, since our common sense is restricted to our daily experience with objects that are not so delicate. What do we mean by acting like a particle? They can be counted. A photon is like a “currency”, and the light that we experience is like a sum of money, we never notice that tiny penny.
Let us take a closer look and see why we generally ignore single photons. A typical red laser pointer has an output power of 3 mW (3 mJ/s), which consists of individual photons having an energy of the order of 3 × 10 –19 J. This means that every second there are 10 000 million million photons shooting out of a pointer. If we associate even a penny to each photon, in a second we get a sum of money that is more than the wealth of a country.
Just as not all currencies have the same value, photons have different energies. Here we need to use the wave aspect of the photon. The faster a wave oscillates, the more energy it possesses.
The longest (slower) electromagnetic wave that we encounter in our daily life is created by the 50 Hz electrical network covering the globe. As a result the earth radiates, making one oscillation over a distance of 6000 km. Radio waves are long too: it takes 3 m (3.3 yd) for a short wave (FM radio) to make an oscillation. For a long wave (AM) it takes about 300 m (330 yd) to complete one.
The visible light that we are used to also oscillates, but much faster. The green visible light consists of photons of 500 nm wavelength; meaning that over a thickness of a sheet of paper (which is 0.1 mm or 0.004 of an inch) it makes 200 oscillations. An x-ray with a wavelength of about 1 nm, oscillates 100 000 times over the same length. It thus appears that the following connection exists: photons that oscillate faster have a shorter wavelength, and more energy. Or in the simplified language of mathematics
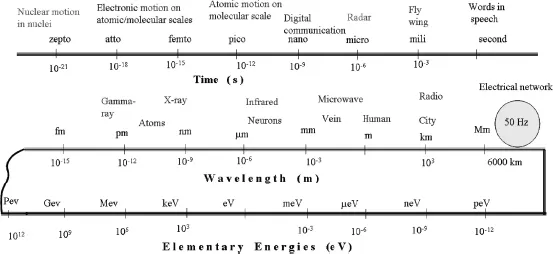
where “E” stands for energy, “h” is the physical Planck’s constant, “c” is the speed of light, “ν” is the number of oscillations of a photon in a second, and “λ” is the wavelength, or the length in which a single oscillation takes place. For the photon associated with visible radiation, the elementary photon energy is too small to use the traditional energy unit of Joule. Instead, the energy unit used by physicists is the electronvolt (eV). 1 eV (1.602 × 10–19 J) is the energy acquired by an electron that is accelerated under the potential difference of 1 V. Infrared radiation at a wavelength of 1.24 μm has exactly the energy of 1 eV. As shown in Figure 1.2, our earth, due to the electric power network, radiates photons of 2.067 × 10 – 13 eV energy.
1.1.3 Energy and Size
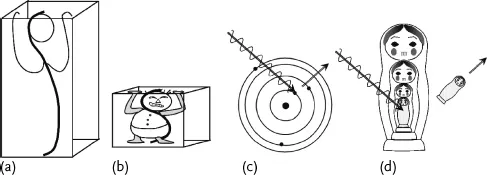
Could “Spiderman” really have the strength of a spider, scaled up to his size? Is a cat that is 100 times more massive than a mouse 100 times stronger? In biology things will not scale linearly. Body mass increases linearly with volume in three dimensions, while muscle strength in arms and legs is proportional to cross-sections, and therefore increases only in two dimensions. If a human is a million times more massive than an ant, he is only 10 000 times stronger. In a way smaller animals are stronger relative to their masses. Physics scales in a simpler way than biology. In a musical instrument higher frequencies are generated by shorter strings, thus have more energy. Some physicists like to draw a box around the object that they study, and they know that as the box gets smaller they are dealing with higher and higher energies. The speed and energy of the electrons oscillating in an atom are much bigger than the ones traveling in a long wire loop.
Using our wave picture and the equation of photon energy (1.1) we can look more closely at the size–energy relation. Consider fitting one full wave into two different size boxes. The wave that fits in the smaller box (Figure 1.3b) has a shorter wavelength than the one in the bigger box (Figure 1.3a). Using the photon energy equation (1.1), the wave with a smaller wavelength has higher energy. It seems that the more confined the wave, the stronger its elementary energy. This seems like an oppression force! Quantum mechanics tells us that the electrons around an atom are confined to well defined shells or electron levels like Russian nesting dolls (Figure 1.3c, d). The electrons in bigger shells have less energy and are loosely bound, which is why in most ionization processes the chance of knocking off an electron from an outer shell is the highest. This order is not as rigid as the order of taking out the Russian dolls: when dealing with higher energies photons, it is possible to scoop up the electron from an internal shell, leaving the external ones in place (not something you could to with the Russian dolls).
Looking at the waves in boxes, we only concentrated on the concepts of s...