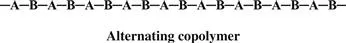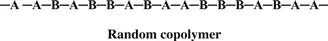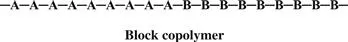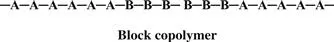1.1 Introduction to Plastics and Polymers
The basic component of plastic and elastomer materials is polymer. The word polymer is derived from the Greek term for “many parts”. Polymers are large molecules composed of many repeat units called monomers that have been chemically bonded into long chains. Since World War II, the chemical industry has developed a large quantity of synthetic polymers to satisfy the materials needs for a diverse range of products, including paints, coatings, fibers, films, elastomers, and structural plastics. Literally thousands of materials can be called “plastics”, although the term today is typically reserved for polymeric materials, excluding fibers, which can be molded or formed into solid or semisolid objects. As of the beginning of 2012, IDES The Plastics Web® (http://www.ides.com) listed over 85,000 different grades of plastic from over 800 suppliers.
There are three introductory chapters to this book. The first chapter is a review of polymer chemistry and plastic formulation. It lays the foundation for the discussion on weather processes, property measurement and all the data chapters. The second chapter is a review of weathering and ultraviolet (UV) light exposure. This includes the various ways to expose test plaques including natural exposures and accelerated exposures. The physical and chemical processes involved with weather and light exposure are explained. The third chapter is on plastic properties. First discussed are the physical properties. Second are the mechanical properties such as tensile strength, elongation, modulus, and tear resistance. Third are thermal properties such as melting point, glass transition temperature and melt index which affect use, production and processing of films.
The chapters that follow are the data chapters. Each chapter covers plastics that fall into particular types based on the chemistry of the polymer. Each of these chapters reviews the chemical structures of the polymers used to make the plastics. In many cases, photochemistry and photodegradation reactions are discussed. Typical stabilizers are mentioned and data are presented in tabular and graphical forms.
The subject of this chapter includes polymerization chemistry and the different types of polymers and how they can differ from each other. Since plastics are rarely “neat”, reinforcement, fillers and additives are reviewed. A basic understanding of plastic and polymer chemistry will make the discussion of properties of specific films easier to understand and it also provides a basis for the introduction of the plastic families in later chapters. This chapter is taken from The Effect of Temperature and Other Factors on Plastics1 and Permeability Properties of Plastics and Elastomers,2 but it has been rewritten, expanded and refocused on polymers as they relate to plastics that may be exposured to various weathering processes.
1.1.1 Polymerization
Polymerization is the process of chemically bonding monomer building blocks to form large molecules. Commercial polymer molecules are usually thousands of repeat units long. Polymerization can proceed by one of several methods. The two most common methods are called addition and condensation polymerization.
1.1.1.1 Addition Polymerization
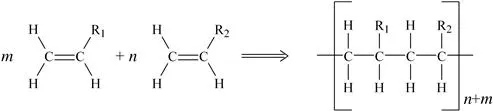
In addition polymerization (sometimes called chain-growth polymerization), a chain reaction adds new monomer units to the growing polymer molecule one at a time through double or triple bonds in the monomer. The polymerization process takes place in three distinct steps:
1. Chain initiation: usually by means of an initiator which starts the polymerization process. The reactive initiation molecule can be a radical (free radical polymerization), cation (cationic polymerization), anion (anionic polymerization) or/and organometallic complex (coordination polymerization).
2. Chain propagation: a monomer adds onto chain and each new monomer unit creates an active site for the next attachment. The net result is shown in Fig. 1.1.
Figure 1.1 Addition polymerization.
3. Chain termination: the radical, cation or anion is “neutralized” stopping the chain propagation.
Many of the plastics discussed in later chapters of this book are formed in this manner. Some of the plastics made by addition polymerization include polyethylene, polyvinyl chloride (PVC), acrylics, polystyrene, and polyoxymethylene (acetal).
1.1.2 Condensation Polymerization
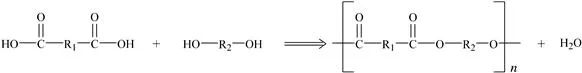
The other common method is condensation polymerization (also called step-growth polymerization) in which the reaction between monomer units and the growing polymer chain end group releases a small molecule, often water, as shown in Fig. 1.2. The monomers in this case have two reactive groups. This reversible reaction will reach equilibrium and halt unless this small molecular by-product is removed. Polyesters and polyamides are among the plastics made by this process.
Figure 1.2 Condensation polymerization.
Understanding the polymerization process used to make a particular plastic gives insight into the nature of the plastic. For example, plastics made via condensation polymerization, in which water is released, can degrade when exposed to water at high temperature. Polyesters such as polyethylene terephthalate (PET) can degrade by a process called hydrolysis when exposed to acidic, basic or even some neutral environments severing the polymer chains. The polymer’s properties are degraded as a result.
1.2 Copolymers
A copolymer is a polymer formed when two (or more) different types of monomers are linked in the same polymer chain, as opposed to a homopolymer where only one monomer is used. If exactly three monomers are used, it is called a terpolymer.
Monomers are only occasionally symmetric; the molecular arrangement is the same no matter which end of the monomer molecule you are looking at. The arrangement of the monomers in a copolymer can be head to tail, head to head, or tail to tail. Since a copolymer consists of at least two types of repeating units, copolymers can be classified based on how these units are arranged along the chain. These classifications include
• Alternating copolymer
• Random copolymer (statistical copolymer)
• Block copolymer
• Graft copolymer
When the two monomers are arranged in an alternating fashion, the polymer is called, of course, an alternating copolymer.
In the following examples, A and B are different monomers. Keep in mind that A and B do not have to be present in a 1:1 ratio. In a random copolymer, the two monomers may follow in any order.
In a block copolymer, all of one type of monomer is grouped together, and all of the second monomer are grouped together. A block copolymer can be thought of as two homopolymers joined together at the ends.
A polymer that consists of large grouped blocks of each of the monomers is also considered a block copolymer.
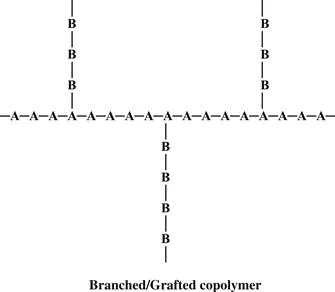
When chains of a polymer made of monomer B are connected onto a polymer chain of monomer A, we have a graft copolymer.
High-impact polystyrene is a graft copolymer. It is a polystyrene backbone with chains of polybutadiene grafted onto the backbone. Polystyrene gives the material strength, but the rubbery polybutadiene chains give it resilience to make it less brittle.
1.3 Linear, Branched and Cross-Linked Polymers
Some polymers are linear, a long chain of connected monomers. Polyethylene, PVC, Nylon 66 and polymethyl methacrylate are some linear commercial examples found in this book. Branched polymers can be visualized as a linear polymer with side chains of the same polymer attached to the main chain. While the branche...