
eBook - ePub
Light Alloys
Metallurgy of the Light Metals
Ian Polmear, David StJohn, Jian-Feng Nie, Ma Qian
This is a test
Partager le livre
- 544 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Light Alloys
Metallurgy of the Light Metals
Ian Polmear, David StJohn, Jian-Feng Nie, Ma Qian
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
Light Alloys: From Traditional Alloys to Nanocrystals, Fifth Edition, covers the materials science, properties, manufacturing processes, and applications of key engineering metals in a single accessible volume. As use of these metals is now more widespread than ever, with routine use in motor vehicles and aircraft, this book includes materials characteristics and applications, heat treatment properties, fabrication, microstructure/property relationships, new applications, and processes.
- Provides a definitive, single volume overview on the light alloys
- Presents new material on the processing, characteristics, and applications of these essential metals
- Covers the latest applications and processes in the auto and aero industries
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Light Alloys est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Light Alloys par Ian Polmear, David StJohn, Jian-Feng Nie, Ma Qian en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Tecnologia e ingegneria et Scienza dei materiali. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
1
The Light Metals
Abstract
This chapter is concerned with introducing and comparing the three most important light metals—aluminum, magnesium, and titanium. Details are given about their discovery, mineralogy, abundance, and production in different countries. Processes are described by which each of these metals can be extracted from their most important minerals. Their relative physical characteristics are compared with other commonly used metals. Aluminum is by far the most used of the three light metals and worldwide production figures are provided. Comparisons are made between the applications of aluminum and its alloys in the advanced and emerging economies. Attention is also given to methods by which these relatively expensive metals can be recycled. Brief reference is also made to the relatively rare light metal beryllium which is the lightest of all the structural elements.
Keywords
Aluminum; magnesium; titanium; metal extraction; physical properties; global production; cost comparisons; recycling; beryllium
1.1 General Introduction
The term “light metals” has traditionally been given to both aluminium and magnesium, because they are frequently used to reduce the weight of components and structures. On this basis, titanium also qualifies and beryllium should be included although it is little used and will only be mentioned briefly later. These four metals have relative densities ranging from 1.7 (magnesium) to 4.5 (titanium) which compare with 7.9 and 8.9 for the older structural metals, iron, and copper, and 22.6 for osmium, the heaviest of all metals. Ten other elements that are classified as metals are lighter than titanium but, with the exception of boron in the form of strong fibers embedded in a suitable matrix, none is used as a base material for structural purposes. The alkali metals lithium, potassium, sodium, rubidium, and cesium, and the alkaline earth metals calcium and strontium are too reactive, whereas yttrium and scandium are comparatively rare.
1.1.1 Characteristics of light metals and alloys
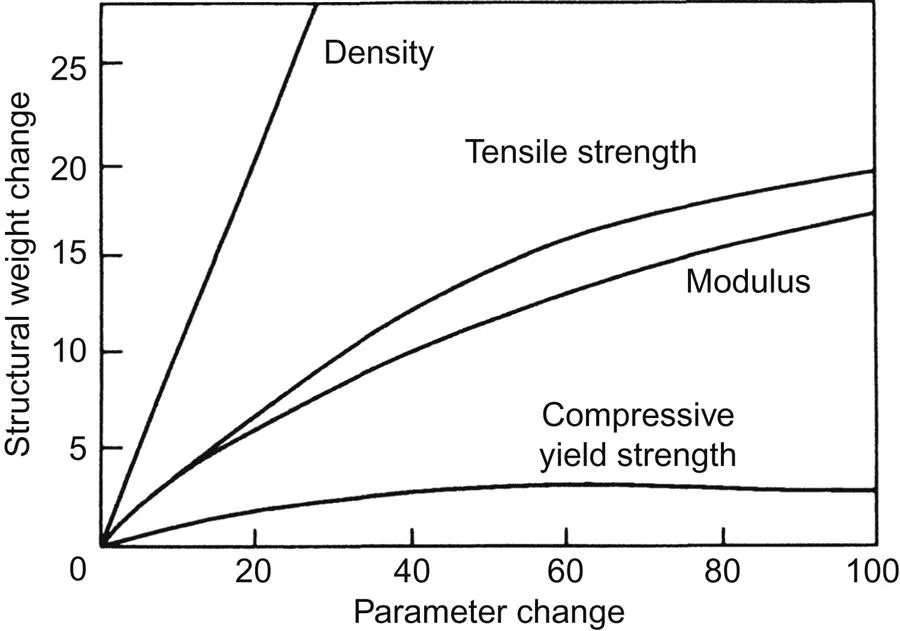
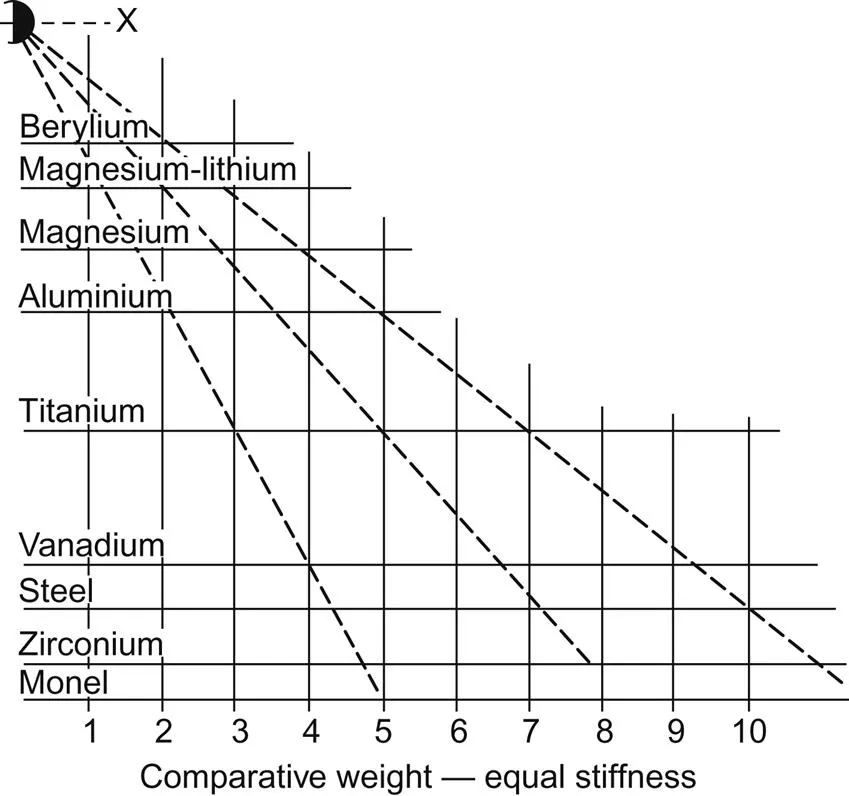
The property of lightness translates directly to material property enhancement for many products since by far the greatest weight reduction is achieved by a decrease in density (Fig. 1.1). This is an obvious reason why light metals have been associated with transportation, notably aerospace, which has provided great stimulus to the development of light alloys during the last century. Strength:weight ratios have also been a dominant consideration and the central positions of the light alloys based on aluminium, magnesium, and titanium with respect both to other engineering alloys and to all materials are represented in an Ashby diagram in Fig. 1.2. The advantages of decreased density become even more important in engineering design when parameters such as stiffness and resistance to buckling are involved. For example, the stiffness of a simple rectangular beam is directly proportional to the product of the elastic modulus and the cube of the thickness. The significance of this relationship is illustrated by the nomograph shown in Fig. 1.3 which allows the weights of similar beams of different metals and alloys to be estimated for equal values of stiffness. An iron (or steel) beam weighing 10 kg will have the same stiffness as beams of equal width and length weighing 7 kg in titanium, 4.9 kg in aluminium, 3.8 kg in magnesium, and only 2.2 kg in beryllium. The Mg–Li alloy is included because it is the lightest (relative density 1.35) structural alloy that is available commercially. Comparative stiffnesses for equal weights of a similar beam increase in the ratios 1:2.9:8.2:18.9 for steel, titanium, aluminium, and magnesium respectively.

Concern with aspects of weight saving should not obscure the fact that light metals possess other properties of considerable technological importance. Examples are the generally high corrosion resistance and high electrical and thermal conductivities of aluminium, the castability and machinability of magnesium, and extreme corrosion resistance of titanium. Comparisons of some physical properties are made in Table 1.1.
Table 1.1
| Property | Unit | Al | Mg | Ti | Be | Fe | Cu |
|---|---|---|---|---|---|---|---|
| Atomic number | − | 13 | 12 | 22 | 4 | 26 | 29 |
| Relative atomic mass (C=12.000) | − | 26.982 | 24.305 | 47.90 | 9.012 | 55.847 | 63.546 |
| Crystal structure | − | fcc | cph | cph | cph | bcc | fcc |
| a | nm | 0.4041 | 0.3203 | 0.2950 | 0.2286 | 0.2866 | 0.3615 |
| c | nm | − | 0.5199 | 0.4653 | 0.3583 | − | − |
| Melting point | °C | 660 | 650 | 1678 | 1289 | 1535 | 1083 |
| Boiling point | °C | 2520 | 1090 | 3289 | 2472 | 2862 | 2563 |
| Relative density (d) | − | 2.70 | 1.74 | 4.51 | 1.85 | 7.87 | 8.96 |
| Elastic modulus (E) | GPa | 70 | 45 | 120 | 295 | 211 | 130 |
| Specific modulus (E/d) | − | 26 | 26 | 26 | 160 | 27 | 14 |
| Mean specific heat 0–100°C | J kg−1 K−1 | 917 | 1038 | 528 | 2052 | 456 | 386 |
| Thermal conductivity 20–100°C | W m−1 K−1 | 238 | 156 | 26 | 194 | 78 | 397 |
| Coefficient of thermal expansion 0–100°C | 10−6 K−1 | 23.5 | 26.0 | 8.9 | 12.0 | 12.1 | 17.0 |
| Electrical resistivity at 20°C | μ ohm cm | 2.67 | 4.2 | 54 | 3.3 | 10.1 | 1.69 |
Note: Conversion factors for S1 and Imperial units are given in the Appendix.
From Lide, DR (Ed.): Handbook of Chemistry & Physics, 72nd Ed., CRC Press, Boca Raton, FL, USA, 1991–92; Metals Handbook, Volume 2, 10th Ed., ASM International, Metals Park, OH, USA, 1990.
1.1.2 Beryllium
This element was discovered by Vauquelin in France in 1798 as the oxide in the mineral beryl (beryllium aluminium silicate) and in emerald. It was first isolated independently by Wöhler and Bussy in 1828 who reduced the chloride with potassium. Beryl has traditionally been a by-product of emerald mining and was until recently the major source of beryllium metal. Currently more beryllium is extracted from the closely associated mineral bertrandite (beryllium silicate hydroxide). Beryllium has some remarkable properties (Table 1.1). Its stiffness, as measured by specific elastic modulus, is nearly an order of magnitude greater than that for the other light metals, or for the commonly used heavier metals iron, copper, and nickel. This has led to its use in gyroscopes and in inertial guidance systems. It has a relatively high melting point, and its capture cross section (i.e., permeability) for neutrons is lower than for any other metal. These properties have stimulated much interest by the aerospace and nuclear industries. For example, a design study specifying beryllium as the major struct...
