
eBook - ePub
Biocontamination Control for Pharmaceuticals and Healthcare
Tim Sandle
This is a test
Partager le livre
- 374 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Biocontamination Control for Pharmaceuticals and Healthcare
Tim Sandle
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
Biocontamination Control for Pharmaceuticals and Healthcare outlines a biocontamination strategy that tracks bio-burden control and reduction at each transition in classified areas of a facility. This key part of controlling risk escalation can lead to the contamination of medicinal products, hence necessary tracking precautions are essential. Regulatory authorities have challenged pharmaceutical companies, healthcare providers, and those in manufacturing practice to adopt a holistic approach to contamination control. New technologies are needed to introduce barriers between personnel and the environment, and to provide a rapid and more accurate assessment of risk. This book offers guidance on building a complete biocontamination strategy.
- Provides the information necessary for a facility to build a complete biocontamination strategy
- Helps facilities understand the main biocontamination risks to medicinal products
- Assists the reader in navigating regulatory requirements
- Provides insight into developing an environmental monitoring program
- Covers the types of rapid microbiological monitoring methods now available, as well as current legislation
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Biocontamination Control for Pharmaceuticals and Healthcare est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Biocontamination Control for Pharmaceuticals and Healthcare par Tim Sandle en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Medicina et Farmacologia. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
Sujet
MedicinaSous-sujet
FarmacologiaChapter 1
Introduction to Biocontamination and Biocontamination Control
Abstract
Biocontamination refers to biological contamination of products by microorganisms and the toxic by-products of these microorganisms. When designing a biocontamination control strategy for a pharmaceutical or healthcare facility, account must be taken of the manufacturing process together with the vital components, each of which requires risk assessment. These include designing process systems to avoid contamination, monitoring process systems to detect contamination, and reacting to contamination events with proactive measures. Process systems design is where maximum effort should be placed. These themes are set out; the chapter additionally serves as an introduction to the book’s contents, outlining the key messages in each chapter.
Keywords
Biocontamination; Environmental monitoring; Disinfection; Microorganisms; Risk assessment; Pharmaceuticals; Healthcare
Introduction
Biocontamination refers to biological contamination of products by bacteria and/or fungi, as well as the toxic by-products of these microorganisms, such as endotoxin and mycotoxins from Gram-negative bacteria and fungi, respectively. This book considers biocontamination within the context of pharmaceuticals and healthcare, with the focus of developing medicinal products that are safe. This level of safety cannot simply be achieved through putting individual protective measures in place and it certainly cannot be achieved through simply monitoring. To achieve the aim of biocontamination control each element needs to be looked at in the connected sense and fitted into a biocontamination control strategy (Sandle, 2015). Such a strategy is a fundamental element of the pharmaceutical quality system. The core points are relevant, to different degrees, to both sterile and nonsterile pharmaceuticals, as well as medical devices and biotechnology products (Sandle, 2013a).
When designing a biocontamination control strategy there are three components that need to be taken into account, and each of which needs to be risk based, drawing on the principles of quality risk management. First, processes need to be designed to avoid contamination. This demands the application of quality by design principles, which will vary according to different types of manufacturing and facilities. Important here is the selection of appropriate technologies, their design, and consideration of how they can best be implemented to minimize contamination and to lower the possibility of cross-contamination occurring. Second, there needs to be a sound monitoring process to detect contamination. Third, there need to be a rapid response to contamination events and for putting proactive measures in place. When considering contamination events, the data from monitoring programs needs to be considered holistically. A breakdown of control downstream or in lower graded cleanrooms can signal later deterioration of control in relation to the product or the environment where the product undergoes final formulation or filling. Of these different elements it is the design of process where maximal effort needs to be placed (Sandle, 2013b).
There is, of course, a role for monitoring, especially once good design principles are in place. Environmental monitoring program should be designed in order to provide information about the state of control of the facility. Yet it remains important that an environmental monitoring does not replace good environmental control (the design of cleanrooms and operational practices); environmental monitoring only provides a “snapshot” of time. Individually counts are rarely significant, but it is the trends over time that are important: as counts, as frequency of incidents, and as microflora. The presence of microflora, such as waterborne bacteria or organisms that are hard to kill with disinfectants, may indicate the breakdown of control (Sandle, 2011).
The requirements for maintaining biocontamination control, together with the core elements of a robust strategy, are presented in the chapters that make up this book. Chapter 2 opens the substantive part of this book with discussion of microbial sources within pharmaceutical and healthcare processing environments. This is important since identification of these sources helps to identify where control is most required.
Contamination within healthcare and pharmaceutical facilities can arise from a number of sources. These may vary depending upon the type of cleanroom, its geographic location, the types of products processed, and so on. Nevertheless, these sources can generally be divided into the following groups: people, water, air and ventilation, surfaces, the transport of items in and out of clean areas. These sources are illustrated in Fig. 1.
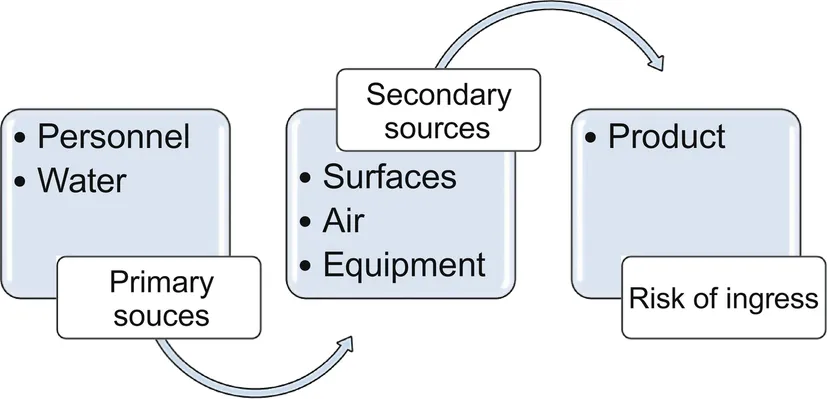
Most contamination within the pharmaceutical facility can be traced to humans working in cleanrooms.
Chapter 3 assesses the regulatory framework, looking at the regulations that are applicable to contamination control (and the differences between them) and the gaps between regulations, identifying the aspects of a control strategy that are not so clear-cut. What the regulations share is that products are developed and manufactured in areas which minimize the potential for contamination. This is through the control of environmental cleanliness and in minimizing the opportunities for personnel to introduce contamination into the process.
Chapter 4 presents the main elements for a biocontamination control strategy. The aim here is to present the key aspects of the strategy and allow those who need to develop such a strategy to mirror the requirements and for those who have a strategy in place to benchmark their practices against. The strategy set out here is risk based (including risk profiling); proactive (in that identified risks need to be addressed); holistic (in seeing each part of the process as interrelated); and which highlights the importance of communication, in that the importance of risk escalation is emphasized.
Chapter 5 considers cleanrooms and the physical and microbiological measurements that can be used to assess cleanroom operations. With cleanrooms, there are a number of physical parameters which require examination on a regular basis. These parameters generally relate to the operation of HVAC systems and the associated air handling system. Air handler, or air handling unit (AHU) relates to the blower, heating and cooling elements, filter racks or chamber, dampers, humidifier, and other central equipment in direct contact with the airflow. Weaknesses or exceptions with any of these areas should be risk assessed and the outcome might lead to variations in the environmental monitoring program (Whyte & Eaton, 2004).
The chapter also assesses the classification and recertification of cleanrooms. The qualification of cleanroom classification is sometimes run as a separate activity to the environmental monitoring program and sometimes it is integral to it. Whichever management model is used, those tasked with routine and batch specific environmental monitoring need to be aware of the outcome of cleanroom classification exercises, including any variations in data and any design issues that are raised.
Chapter 6 looks at viable microbiological monitoring methods, with a focus on environmental monitoring. While these methods are commonly described in text books the limitations with the methods and their variabilities are too often overlooked. Understanding the weaknesses with the methods helps to lower expectations of what can be discerned from the data and helps focus the mind on the importance of environmental control. The methods can be strengthened through assessment and qualification, and the chapter provides some guidance over how each of the core methods can be evaluated.
Following on from Chapter 6, the seventh chapter looks at culture media. This is relatively ill defined in terms of assessing pharmaceutical environments. Here the key questions are Which culture media to use? Should one or two culture media be used? What is the incubation time? What is the appropriate temperature? The chapter assesses key studies that attempt to answer these questions, some of which are better designed than others, and puts together a framework for the optimal use of culture media. Growth promotion requirements are also covered in the chapter.
The nature of particle counting is based upon either light scattering, light obscuration, or direct imaging, and variations inform about control breakdowns. Chapter 8 addresses particle counting, as required for cleanroom classification and ongoing monitoring. The chapter considers the selection criteria for particle counters and some of the specifications which need to be evaluated, such as sensitivity (the smallest size particle that can be detected); false count rates; counting efficiency (the ratio of the measured particle concentration to the true particle concentration, which is typically at 50%); channels, in relation to differential and cumulative counting; and flow rate (the amount of air that passes through the particle counter).
Chapter 9 considers rapid and alternative microbiological methods and what these can offer biocontamination control, especially in relation to faster and more accurate responses, as well as reacting to events in real time. There are an array of different rapid microbiological methods, each with their own technologies and testing protocols, at different levels of maturity. The test methods are grouped in the chapter into the following three categories according to their uses: qualitative, quantitative, and identification.
When assessing alternative methods, data integrity is an important requirement. Data integrity concerns arise at the design, validation, and operation stages. Taking validation, samples need to be representative of what will be tested using the instrument and tested multiple times and by different technicians in order to build in repeatability and robustness. Aspects that give validity to the result, such as limit of detection and limit of quantification (either directly in relation to microorganisms or indirectly through monitoring biological events) need to be introduced.
In terms of operations, data integrity extends to data capture, retention, archiving,...