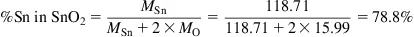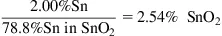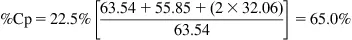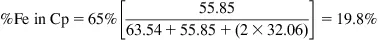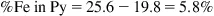1.1 Minerals
The forms in which metals are found in the crust of the earth and as seabed deposits depend on their reactivity with their environment, particularly with oxygen, sulfur, and carbon dioxide. Gold and platinum metals are found principally in the native or metallic form. Silver, copper, and mercury are found native as well as in the form of sulfides, carbonates, and chlorides. The more reactive metals are always in compound form, such as the oxides and sulfides of iron and the oxides and silicates of aluminum and beryllium. These naturally occurring compounds are known as minerals, most of which have been given names according to their composition (e.g., galena—lead sulfide, PbS; cassiterite—tin oxide, SnO2).
Minerals by definition are natural inorganic substances possessing definite chemical compositions and atomic structures. Some flexibility, however, is allowed in this definition. Many minerals exhibit isomorphism, where substitution of atoms within the crystal structure by similar atoms takes place without affecting the atomic structure. The mineral olivine, for example, has the chemical composition (Mg,Fe)2SiO4, but the ratio of Mg atoms to Fe atoms varies. The total number of Mg and Fe atoms in all olivines, however, has the same ratio to that of the Si and O atoms. Minerals can also exhibit polymorphism, different minerals having the same chemical composition, but markedly different physical properties due to a difference in crystal structure. Thus, the two minerals graphite and diamond have exactly the same composition, being composed entirely of carbon atoms, but have widely different properties due to the arrangement of the carbon atoms within the crystal lattice.
The term “mineral” is often used in a much more extended sense to include anything of economic value that is extracted from the earth. Thus, coal, chalk, clay, and granite do not come within the definition of a mineral, although details of their production are usually included in national figures for mineral production. Such materials are, in fact, rocks, which are not homogeneous in chemical and physical composition, as are minerals, but generally consist of a variety of minerals and form large parts of the earth’s crust. For instance, granite, which is one of the most abundant igneous rocks, that is, a rock formed by cooling of molten material, or magma, within the earth’s crust, is composed of three main mineral constituents: feldspar, quartz, and mica. These three mineral components occur in varying proportions in different parts of the same granite mass.
Coals are a group of bedded rocks formed by the accumulation of vegetable matter. Most coal-seams were formed over 300 million years ago by the decomposition of vegetable matter from the dense tropical forests which covered certain areas of the earth. During the early formation of the coal-seams, the rotting vegetation formed thick beds of peat, an unconsolidated product of the decomposition of vegetation, found in marshes and bogs. This later became overlain with shales, sandstones, mud, and silt, and under the action of the increasing pressure, temperature and time, the peat-beds became altered, or metamorphosed, to produce the sedimentary rock known as coal. The degree of alteration is known as the rank of the coal, with the lowest ranks (lignite or brown coal) showing little alteration, while the highest rank (anthracite) is almost pure graphite (carbon).
While metal content in an ore is typically quoted as percent metal, it is important to remember that the metal is contained in a mineral (e.g., tin in SnO2). Depending on the circumstances it may be necessary to convert from metal to mineral, or vice versa. The conversion is illustrated in the following two examples (Examples 1.1 and 1.2).
Example 1.1
Given a tin concentration of 2.00% in an ore, what is the concentration of cassiterite (SnO2)?
Solution
Step 1: What is the Sn content of SnO2?
Molar mass of Sn (MSn) 118.71 g mol−1
Molar mass of O (MO) 15.99 g mol−1
Step 2: Convert Sn concentration to SnO2
Example 1.2
A sample contains three phases, chalcopyrite (CuFeS2), pyrite (FeS2), and non-sulfides (containing no Cu or Fe). If the Cu concentration is 22.5% and the Fe concentration is 25.6%, what is the concentration of pyrite and of the non-sulfides?
Solution
Note, Fe occurs in two minerals which is the source of complication. The solution, in this case, is to calculate first the % chalcopyrite using the %Cu data in a similar manner to the calculation in Example 1.1 (Step 1), and then to calculate the %Fe contributed by the Fe in the chalcopyrite (Step 2) from which %Fe associated with pyrite can be calculated (Step 3).
Molar masses (g mol−1): Cu 63.54; Fe 55.85; S 32.06
Step 1: Convert Cu to chalcopyrite (Cp)
Step 2: Determine %Fe in Cp
Step 3: Determine %Fe associated with pyrite (Py)
Step 4: Convert Fe to Py (answer to first question)