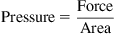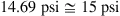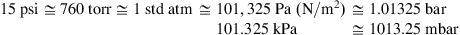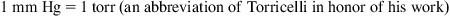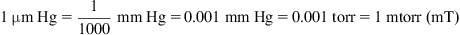Vacuum Deposition onto Webs, Films and Foils
Charles Bishop
- 544 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
Vacuum Deposition onto Webs, Films and Foils
Charles Bishop
À propos de ce livre
Roll-to-roll vacuum deposition is the technology that applies an even coating to a flexible material that can be held on a roll and provides a much faster and cheaper method of bulk coating than deposition onto single pieces or non-flexible surfaces, such as glass.
This technology has been used in industrial-scale applications for some time, including a wide range of metalized packaging (e.g. snack packets). Its potential as a high-speed, scalable process has seen an increasing range of new products emerging that employ this cost-effective technology:
- solar energy products are moving from rigid panels onto flexible substrates, which are cheaper and more versatile
- in a similar way, electronic circuit 'boards' can be produced on a flexible polymer, creating a new range of 'flexible electronics' products
- flexible displays are another area of new technology in vacuum coating, with flexible display panels and light sources emerging
Charles Bishop has written this book to meet the need he identified, as a trainer and consultant to the vacuum coatings industry, for a non-mathematical guide to the technologies, equipment, processes and applications of vacuum deposition. His book is aimed at a wide audience of engineers, technicians and production management. It also provides a guide to the subject for sectors in which vacuum deposition is a novel technology, such as solar energy and flexible electronics.
- Bishop's non-mathematical explanation of vacuum deposition technologies will empower a wide range of technicians, production managers and engineers in related disciplines to improve performance and maximize productivity from vacuum coating systems
- Provides the knowledge and understanding needed to specify systems more effectively and enhance the dialogue between non-specialists and suppliers / engineers
- Provides those in the rapidly expanding fields of solar energy, display panels and flexible electronics with the know-how to unlock the potential of vacuum coating to transform their processes and products
Foire aux questions
Informations
1.1. What Is a Vacuum?
1.2. What Is a Gas?
1.3. Pressure