
eBook - ePub
Principles and Practice of Modern Chromatographic Methods
Kevin Robards, P. E. Jackson, Paul A. Haddad
This is a test
Partager le livre
- 495 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Principles and Practice of Modern Chromatographic Methods
Kevin Robards, P. E. Jackson, Paul A. Haddad
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
Though many separation processes are available for use in todays analytical laboratory, chromatographic methods are the most widely used. The applications of chromatography have grown explosively in the last four decades, owing to the development of new techniques and to the expanding need of scientists for better methods of separating complex mixtures. With its comprehensive, unified approach, this book will greatly assist the novice in need of a reference to chromatographic techniques, as well as the specialist suddenly faced with the need to switch from one technique to another.
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Principles and Practice of Modern Chromatographic Methods est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Principles and Practice of Modern Chromatographic Methods par Kevin Robards, P. E. Jackson, Paul A. Haddad en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Ciencias físicas et Química analítica. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
Sujet
Ciencias físicasSous-sujet
Química analítica1
Introduction and Overview
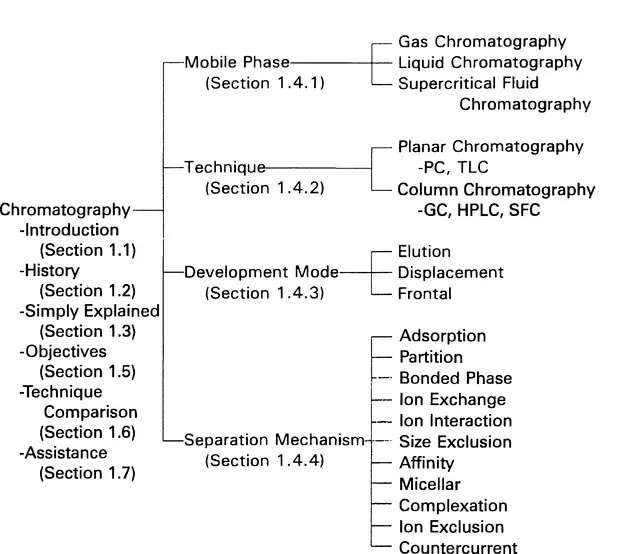
1.1 Introduction
‘What is chromatography?’ This is a logical and seemingly simple question. Chromatography was originally developed by the Russian botanist M. S. Tswett (1872–1919) as a technique for the separation of coloured plant pigments (see Fig. 1.1). Tswett gave a very pragmatic definition [1]. The first detailed definition appears to be by Zechmeister [2] and various subsequent definitions have since been formulated. These definitions are, of themselves, unimportant. What is of interest is that with each successive definition the criteria for a process to be called chromatography have generally been liberalized. A generalized definition was provided by a special committee of the International Union of Pure and Applied Chemistry [3] which regards chromatography as ‘… a method, used primarily for separation of the components of a sample, in which the components are distributed between two phases, one of which is stationary while the other moves. The stationary phase may be a solid, liquid supported on a solid, or a gel. The stationary phase may be packed in a column, spread as a layer, or distributed as a film…. The mobile phase may be gaseous or liquid.’
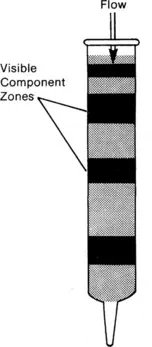
Fig. 1.1 System as used by Tswett in his original experiments. Prior to 1935 the column packing was removed from the column after use and the separated zones were extracted in order to recover the ‘pure’ components.
This definition neglects the possibility of using a supercritical fluid as the mobile phase, which highlights the difficulties associated with providing an adequate definition. Nevertheless, we should not allow ourselves to be distracted by the need for a clear and concise definition, but rather regard chromatography as a group of separation methods that are undergoing continuous development and refinement.
The origins of the word ‘chromatography’ are no less obscure [4, 5]. In Tswett’s papers, it was coined by combining two Greek words, chroma, ‘colour’ and graphein, ‘to write’ selected to indicate the individual coloured bands observed by Tswett in his separations. At the same time Tswett emphasized that colourless substances can be separated in the same way. However, it may well be that Tswett, who was involved in a bitter controversy with his peers, gave reference to the Greek words only as an excuse, for as Purnell [6] states ‘… it would be nice to think that Tswett, whose name, in Russian, means colour, took advantage of the opportunity to indulge his sense of humour.’
Irrespective of such considerations chromatography is a universal and versatile technique. It is equally applicable in all areas of chemistry and biochemistry, biology, quality control, research, analysis, preparative-scale separations and physicochemical measurements. It can be applied with equal success on the macro and micro scale. Chromatography is used industrially in the purification of such diverse materials as cane sugar, pharmaceuticals and rare earths. It is also widely used in the laboratory for the separation of minute quantities of substance, as in the initial chromatographic experiments leading to the discovery of element number 100, which involved only about 200 atoms; this surely represents one of the most remarkable achievements of modern science [7].
The importance of chromatography in science can be illustrated in a number of ways. One of these is the number of publications involving chromatography relative to the total number of science-based publications. Such a comparison shows that 3.3% or a total of approximately 8100 of citations appearing in Chemical Abstracts for 1989 involve chromatography, a significant proportion for any one technique. However, this is an underestimate as a significant number of citations will have exploited chromatography as a technique but will not have referred to it in either the title or abstract. A further illustration is the fact that two Nobel Prizes in Chemistry (to A. Tiselius of Sweden in 1948 and to A. J. P. Martin and R. L. M. Synge of Great Britain in 1952) were awarded for work directly in the field of chromatography. In addition, chromatography played a vital role in work leading to the award of a further twelve Nobel Prizes between 1937 and 1972 [8].
Sales of chromatographic and related equipment have led the field in analytical instrument sales for some years and still represent an expanding market. This is illustrated by the following extract from Trends in Analytical Chemistry [1991, 10: V]: ‘For the 1990s shipments of Chromatographic instruments—the leading product category—are projected to increase in dollar terms from $725 million in 1990 to $990 million in 1994. Included are analytical gas, liquid, ion and supercritical fluid chromatographs as well as detectors employed in these instruments. Columns, supplies and accessories or preparatory chromatographic systems are not included.’ However, the last of these is a rapidly expanding market. Thus, there is a vast amount of chromatography being performed both in academia and industry.
1.2 Historical Aspects
There is a temptation to ignore the work of the past. However, in order to exploit fully current developments, an awareness of past advances is desirable. A brief historical excursion at this point should place in perspective the development of thought and activity in a technique that has had an important fundamental and applied influence in chemistry and in other areas. This discussion represents an overview rather than a detailed and comprehensive account of the evolution of thought and practice in each chromatographic technique. The reader interested in a more detailed account of the history of chromatography is referred to the various articles published since 1970 [7–12] and to the text by Zechmeister and Cholnoky [13], although this may be difficult to obtain.
Credit for the introduction of chromatography is difficult to assign and is perhaps of academic interest only. Many natural processes, such as the underground migration of fluids through clays and sediments, can be regarded as chromatographic processes. Various ancient texts describe procedures that undoubtedly involve the unconscious use of chromatography. An instance is the conversion, described by Aristotle, of salty and bitter water into potable water with clay. However, credit for the invention of chromatography is not attributed to the observation of these natural processes. Certain investigations in the second half of the last century may be regarded as the precursors of chromatography. Prominent among these is the work of the American petroleum chemist D. T. Day (1859–1925). Zechmeister and Cholnoky [13] first drew attention to Day’s activities. This was followed by a heated exchange of ideas [14–17] culminating in claims that Day was the inventor of chromatography. Significant though Day’s contributions were, his role in the development of chromatography has probably been exaggerated. Indeed, the Editorial Board of the journal Biokhimiya [1951, 16: 478] regarded these claims as a Western plot to ‘reduce the merit of the Russian scientist M. S. Tswett.’ The principles of chromatography as practised nowadays were first outlined by Tswett (for details of his life see, for example, [19–21]) on March 21, 1903 (March 8 according to the old Russian calendar in use at that time) at a meeting of the Biological Section of the Warsaw Society of Natural Sciences. This represents the first report of Tswett’s systematic investigation of the chromatographic separation of plant pigments. Tswett soon became embroiled in a bitter controversy regarding his procedure, which was rejected by his contemporaries [22] as being of little merit. Kohl, an authority on carotene pigments, cited Tswett’s failure to reference Kohl’s own book as proof of the inaccuracy of Tswett’s results [23]. However, Tswett was so aware of the importance and scope of his discovery that he insisted, although his experiments did not result in isolation of pure substances, against all opposition that chlorophyll was a mixture of two components.
Tswett’s involvement with chromatography had almost ceased by 1912 and there followed a 20-year period of dormancy in which f...