
eBook - ePub
Prevention and Combating Mine Fires
Sudhish Chandra Banerjee
This is a test
Partager le livre
- 384 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Prevention and Combating Mine Fires
Sudhish Chandra Banerjee
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
There is a growing concern about fires in mines, coal mines in particular. Attempts are being made to improve mine fire combat efforts and their prevention, taking advantage of developments in the fields of science and technology. This book looks at those developments and their applications.
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Prevention and Combating Mine Fires est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Prevention and Combating Mine Fires par Sudhish Chandra Banerjee en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Technik & Maschinenbau et Bauingenieurwesen. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
CHAPTER 1
The Science of Fires
“Pinğabhrū Śmaśru KésākṣaḥPīnānğa JaṭharóruṇaḥChāgasthaḥ SāksyasūtrógniḥSaptārcciḥ Saktidhārakaḥ”
A huge bodied, red bellied persona with three tier tassles of golden yellow—the eyebrows, beards and hairs—being seated over the sacrificial being and garlanded with beads of purity, forms the emblem of power and energy, the Fire-God with its effulgent glare of the seven*—the fierce, the fumes, the niveous, the red, the crimson, the golden and the lectious like pollens of lotus.
That is how the ancient Seers of India, who used to personify everything in nature, portrayed fire as the Fire-God.
Such aesthetic depiction of fire by ancient Indians, of course conforms fairly well with our present day knowledge on the attributes of fire. The fire plume of most of the natural fires do have three distinct regions of golden yellow leaping flames, as shown in Fig. 1. Besides the flame and the smoke that helps in spreading the ferocity of fire, the object so affected by fire may have glares of different shades of colours depending on intensity as given in Table 1.
Temperature (°C) | Appearance |
|---|---|
550 | First Visible red glow |
700 | Dull red |
900 | Cherry red |
1100 | Orange |
1400 | White |
In fact since the dawn of civilisation, man’s efforts to understand fire have been directed at taming it in order to harness heat and light for the benefit of mankind and to control its unchecked advancement. Different disciplines of science has contributed on this topic since ages and the subject of fire science has assumed an interdisciplinary character. In the context of mine fires, the focus is on understanding the principle of occurrence of fire, nature of propagation with a view to its control and suppression.
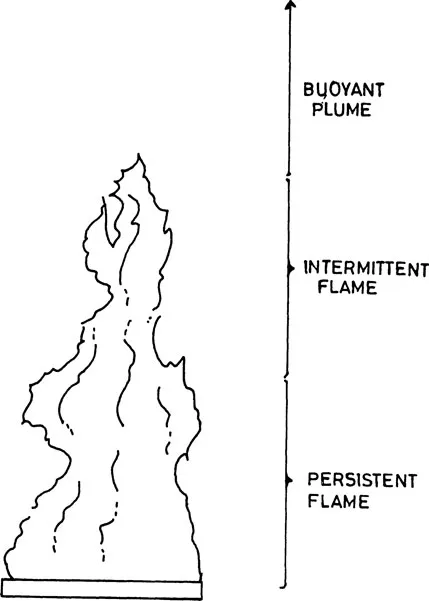
PRINCIPLES OF OCCURRENCE OF FIRE
Fuels, whether in solid, liquid or gaseous form, may undergo rapid chemical change interacting with oxygen or oxidants releasing large amount of energy at a fast rate that manifests in the form of heat and light. This manifestation is known as fire and the process is said ignition or combustion or burning of fuel. The initiation of such ignition may be of two types viz.,
• piloted ignition, where inputs of external energy (electric spark, independent flame, etc.) is required for the release of energy from fuel that would be sufficient for the exothermic reaction to be self sustaining and thereby propagate the fire (Exogenous Fires).
• Spontaneous ignition, where the heat flux generated from spontaneous heating is sufficiently high to ignite the volatiles released from the fuel bed (Endogenous fires).
In fact, such type of spontaneous ignition requires a higher heat flux than piloted ignition. There is also a lag phase for spontaneous ignition to burst out in flames.
It may be noted that all types of fires associated with flames—in cases of both piloted and spontaneous ignition—are always a gas phase phenomenon. Flaming combustion involving both solid and liquid fuels must precede their conversion into gaseous form. In case of liquids it may be simple evaporation, whence for solid fuels chemical decomposition or pyrolysis is necessary to yield products of low molecular weights that can volatalise from surface and flare up with flames. As this decomposition process requires more energy than simple evaporation, surface temperature of a burning solid tend to be rather high, 400°C or above - excepting of course for solids that directly sublime on heating. The release of gaseous products from solids may either be from direct sublimation of the solid or, evaporation via melting-cum-decomposition or, from direct decomposition/pyrolysis yielding volatiles of low molecular weight.
Certain polymers have the characteristics of decomposing to yield products that may resist further flaming combustion. A typical example is the thermal decomposition of PVC (polyvinyl chloride), which lose molecular HCl at about 250°C and yield a char like residue e.g.
The charred product formed is difficult to ignite, other than at high temperature. Usually polymers which undergo cross linking during pyrolysis, tend to char on heating—requiring much hig...