![]()
The Future
![]()
Chapter 13
Donald PR, van Helden PD (eds): Antituberculosis Chemotherapy.
Prog Respir Res. Basel, Karger, 2011, vol 40, pp 118-127
______________________
Issues and Challenges in the Development of Novel Tuberculosis Drug Regimens
Ngozi Erondu · Ann Ginsberg
Global Alliance for TB Drug Development, New York, N.Y., USA
______________________
Abstract
Due to the limitations of current tuberculosis (TB) treatment, there is an urgent need for the development of novel TB drug regimens to address the persistent and worsening global TB epidemic. The main goals for developing new regimens are: shortening and simplifying the treatment of active, drug-sensitive and drug-resistant TB; effectively treating TB in patients co-infected with HIV with drugs that do not adversely interact with antiretroviral agents, and shortening the treatment of latent TB infection. Key challenges for novel regimen development include the need for multiple safe, efficacious drugs with novel mechanisms of action, and the long duration and high cost of TB clinical trials. There are now 9 drugs in various stages of clinical development, belonging to 6 different chemical classes with distinct mechanisms of action. This represents the most active pipeline for TB drug development in history. The status of the clinical development of these drugs - AZD5847, PNU-100480, rifapentine, PA-824, delamanid, TMC207, SQ109, moxifloxacin and gatifloxacin - will be reviewed in this chapter. To significantly reduce the long development timelines, a new paradigm, which considers the whole multidrug combination rather than the individual drug as the unit of clinical development and target for registration, has been proposed and will also be discussed.
Copyright © 2011 S. Karger AG, Basel
Limitations and inadequacies of the current tuberculosis (TB) treatment regimens contribute significantly to the global burden of TB, the emergence and spread of multidrugresistant (MDR) and extensively drug-resistant TB, and the difficulty of controlling this disease and reaching the Millennium Development Goals and Stop TB Partnership global targets for reducing disease burden. Current TB treatment regimens are described in detail elsewhere in this volume. However, some key features include the following: (1) adequate treatment of drug-sensitive TB requires the use of a 4-drug combination (1-letter abbreviations will be used for combinations as follows: H = isoniazid, R = rifampicin, Z = pyrazinamide and E = ethambutol) for 6-9 months; (2) the rifamycins, a key component of first-line TB treatment, have significant interactions with the cytochrome (CYP) P450 system; specifically, they cause the induction of enzymes/transporters that are involved in the metabolism of several drugs including some antiretroviral agents, which makes the treatment of TB patients co-infected with HIV challenging, especially in resource-limited settings; (3) the long duration of therapy and the side effects of the current drugs lead to non-compliance, a major contributor to the emergence and spread of drug-resistant TB, treatment of which often lasts for 2 or more years and requires more expensive drugs (including injectables) with limited or uncertain efficacy and worse safety profiles; (4) treatment of latent TB, key to controlling the epidemic especially in regions of the world with high HIV infection rates, currently requires lengthy treatment (up to 9 months) with isoniazid although shorter regimens [e.g. 3 months of weekly rifapentine (P) + isoniazid] are being evaluated (see below; NCT00023452).
Given the limitations and challenges described above, the need for the development of new treatment regimens of both active and latent TB cannot be overemphasized. The ultimate goals are to develop a regimen for active TB that can shorten and simplify the treatment of both drug-sensitive (DS) and MDR-TB in both HIV-infected and uninfected individuals, and an ultrashort, simple, safe regimen to eradicate latent TB infection. The optimal or ideal target profile of drugs in such novel regimen(s) includes:
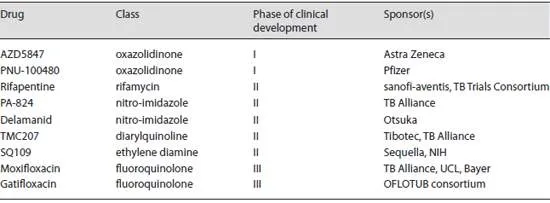
Table 1. Drugs in clinical development
• novel mechanism of action with equal efficacy against both DS- and MDR-TB;
• no cross-resistance with current drugs, minimizing the likelihood of pre-existing resistance;
• no significant cytochrome P450-mediated drug-drug interactions, thereby allowing safe and effective coadministration with antiretroviral agents;
• good pharmacokinetic profile in both adult and paediatric populations reflected by being able to be administered orally, once daily or less frequently, and suitability for development in a fixed dose combination;
• excellent safety and tolerability profile;
• low cost of goods, making the regimen affordable in resource-limited regions of the world where TB is highly prevalent.
Drugs in Clinical Development
As shown in table 1 , there are at least 9 drugs currently in various stages of clinical development. Compared to currently approved anti-TB medications, all these drugs with the exception of rifapentine have novel mechanisms of action and are active against both DS- and MDR-TB.
Phase I
There are 2 drugs in this phase of development, both poised to enter phase II in the near future. AZD5847 and PNU-100480 both belong to the oxazolidinone class of antimicrobial agents and are being developed by Astra Zeneca and Pfizer, respectively. The mycobactericidal activity of these drugs results from inhibition of protein synthesis. The prototype for this class, linezolid, although used off label for the treatment of MDR-TB, is hampered by a side effect profile that includes haematological and neurological toxicity.
PNU-100480 (PNU) is derived from linezolid but is more potent and appears to have a better safety profile. In the mouse model of TB infection [1 ], it has been shown that PNU is more bactericidal than linezolid and causes a 2-log reduction in lung CFU counts when added to standard therapy (HRZ) or a 2-drug regimen such as moxifloxacin (M) and pyrazinamide. Interestingly, PNU + MZ was more active than HRZ. In another mouse study, PNU had better sterilizing activity than linezolid, and when combined with HRZ, shortened the duration of treatment required for cure [2 ]. These data support the potential of a PNU-containing regimen to result in treatment shortening for both DS- and MDR-TB. A number of studies with PNU in healthy volunteers have been completed, including single and multiple-dose studies as well as assessment of food effect and bactericidal activity using the whole-blood assay. Available data indicate that PNU was well tolerated in single doses up to 1,500 mg and multiple doses up to 600 mg b.i.d. for 28 days. Its mycobactericidal activity determined with the whole-blood assay was superior to that of linezolid, and for both drugs, the maximum activity occurred at ≥ 2 times the minimum inhibitory concentration [3 ]. Simulations using PNU as monotherapy indicate that optimal efficacy would require a total daily dose of 800-1,200 mg [4 ]. The next planned study is a phase II extended early bactericidal activity (eEBA) study in patients with pulmonary TB.
The other oxazolidinone in early development is AZD5847. A single-dose phase I study in healthy volunteers, which included the assessment of food effects, has been completed while a 14-day multiple ascending dose study to assess the safety, tolerability and pharmacokinetics of an oral suspension is ongoing (NCT01116258). No data have been publicly released.
Phase II
The 5 drugs in this phase are: rifapentine, a rifamycin with a long half-life being evaluated for use with currently available drugs by sanofi-aventis in collaboration with the TB Trials Consortium of the US Centers for Disease Control; PA-824 and delamanid, 2 nitro-imidazoles being developed by the TB Alliance and Otsuka, respectively; TMC207, a diarylquinoline being jointly developed by Tibotec (for MDR-TB) and the TB Alliance (for DS-TB), and SQ109, an ethylene diamine being developed by Sequella Inc., in collaboration with the National Institutes of Health.
Rifapentine was approved for the treatment of TB in the USA in 1998 but based on data from patients and the mouse model of TB infection, the currently approved dosing recommendation (600 mg twice weekly) may not be optimal. It appears that substantially superior efficacy may be achieved by administering higher doses of the drug, potentially enabling a significant reduction in treatment duration [5 ]. In addition, multidrug regimens containing rifapentine, e.g. PMZ and TMC207 + PZ, have been shown to be highly effective in mice infected with Mycobacterium tuberculosis [6 , 7 ]. There are several ongoing clinical studies with rifapentine including: a 14-day study evaluating the pharma-cokinetics, safety and tolerability of escalating doses (up to 20 mg/kg) in healthy volunteers (NCT01162486); an 8-week study comparing the efficacy, safety and tolerability of daily rifapentine (approx. 10 mg/kg/dose) to that of daily rifampicin (approx. 10 mg/kg/dose) when added to HZE in patients with pulmonary TB (NCT00694629); an 8-week study comparing the efficacy, safety and tolerability of the experimental regimen HPMZ to the standard therapy (HRZE) in patients with pulmonary TB (NCT00728507); a non-inferiority study (Rifaquin) comparing 2 experimental regimens containing high-dose rifapentine (15 mg/kg body weight, 2 months of daily EMRZ followed by 2 months of MP given twice weekly; 20 mg/kg body weight, 2 months of daily EMRZ followed by 4 months of MP given once weekly) to the standard regimen (2 months of daily HRZE followed by 4 months of daily HR) in the treatment of pulmonary TB, and a study comparing the efficacy, safety and tolerability of a 3-month course of weekly rifapentine and isoniazid (3HP) to a 9-month course of daily isoniazid (9H) in preventing TB among high-risk tuberculin skin test reactors, including children and HIV-infected persons, who require treatment for latent TB infection (NCT00023452). Preliminary results presented at the International Union against Tuberculosis and Lung Disease (IUATLD) meeting in November 2010 from the latent TB infection study (TB Trials Consortium Study 26) indicate that 3 months of weekly HP was non-inferior to 9 months of daily isoniazid and the completion rate was significantly higher in the 3HP group compared to the 9H group (81.9 vs. 69.5%). However, the use of rifapentine will be limited to DS-TB due to its cross-resistance with rifampicin, and its use in TB patients co-infected with HIV will be complicated by cytochrome P450-mediated drug-drug interactions with antiretroviral agents.
PA-824 is a nitro-imidazo-oxazine with anti-TB activity and a unique mechanism of action which has not yet been fully defined. It has been demonstrated to inhibit mycobacterial protein and lipid synthesis, and to inhibi...