![]()
Camacho-Hübner C, Nilsson O, Sävendahl L (eds): Cartilage and Bone Development and Its Disorders.
Endocr Dev. Basel, Karger, 2011, vol 21, pp 1-11
______________________
Chondrocyte Proliferation and Differentiation
Manuela Wuelling · Andrea Vortkamp
Center for Medical Biotechnology, Department of Developmental Biology, University Duisburg- Essen, Essen, Germany
______________________
Abstract
The skeletal elements of the axial and appendicular skeleton are preformed as cartilage templates by a mechanism called endochondral ossification. During this process, a cartilage template is formed in which chondrocytes proliferate and differentiate into hypertrophic chondrocytes and are gradually replaced by bone. Postnatally, remnants of embryonic chondrocytes remain in a restricted domain between the ossified regions of the bones forming the growth plate. The coordinated proliferation and differentiation of chondrocytes ensures the continuous elongation of the epiphyseal growth plates. The sequential changes between proliferation and differentiation are tightly regulated by secreted growth factors, which activate chondrocyte-specific transcription factors. Transcription factors that play critical roles in regulating cell type-specific gene expression include SOX9, GLI2/3 and RUNX2. This review will outline recent advances in the analysis of the complex transcriptional network that regulates distinct steps of chondrocyte differentiation.
Copyright © 2011 S. Karger AG, Basel
Endochondral Ossification
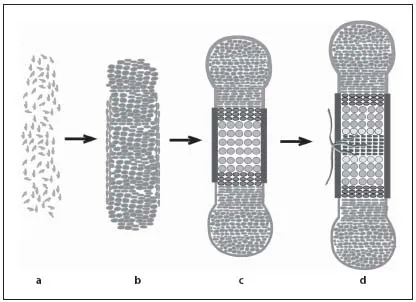
During endochondral ossification, bones are formed by two processes. During intramembranous ossification, by which the bones of the skull and part of the facial bones and clavicle are formed, mesenchymal cells directly differentiate into osteoblasts. In contrast, the axial and appendicular skeleton of vertebrates develops by endochondral ossification. During this process, the initial anlagen are formed as cartilage templates, which model the skeletal elements. Gradually, the cartilage templates are replaced by bone. The process of endochondral ossification starts with the condensation of mesenchymal cells, which differentiate into chondrocytes (fig. 1a, b). These cells initially proliferate and differentiate into two subpopulations of proliferating chondrocytes: round, low proliferating chondrocytes at the distal ends of the condensation (RP chondrocytes) and high proliferating chondrocytes that are organized in columns (CP chondrocytes) towards the center [1–3]. In the center of the growing cartilage anlagen, chondrocytes exit the cell cycle and differentiate into prehypertrophic and hypertrophic chondrocytes (fig. 1c, 2a). These produce a mineralized extracellular cartilage matrix that serves as a template for the subsequent deposition of bone matrix. The increasing volume of the hypertrophic cells contributes significantly to the elongation of the skeletal elements.
Fig. 1. Endochondral ossification requires sequential differentiation and degradation of chondrocytes. Mesenchymal cells condense to form the cartilage anlagen (a) and differentiate into proliferating chondrocytes and fibroblast-like cells in the perichondrium (b). c Chondrocytes form distinct subpopulations, which can be morphologically distinguished from distal to central into RP and CP chondrocytes. After cell cycle exit, chondrocytes differentiate into prehypertrophic, hypertrophic and terminal hypertrophic cells. Adjacent to the hypertrophic zone, cells in the perichondrium mineralize and form the periosteum. d Blood vessels invade the hypertrophic region and initiate bone formation in the center of the skeletal elements.
The cartilage elements are surrounded by a layer of fibroblast-like cells, the perichondrium. At stages when chondrocytes become hypertrophic, cells in the perichondrium differentiate into osteoblasts forming the periost, which is highly vascularized. Blood vessels from the periost invade the calcified matrix of terminal hypertrophic chondrocytes, which undergo apoptosis. Together with the blood vessels osteoblasts and osteoclasts invade the skeletal elements to replace the mineralized cartilage by bone generating the primary spongiosa (fig. 1c, d) [4, 5]. In parallel, osteoblasts in the periost produce a calcified matrix forming the bone collar. The early bone collar and the primary trabecles are later remodeled to form the cortical bone and the bone marrow cavity, which provides a local environment for hematopoiesis.
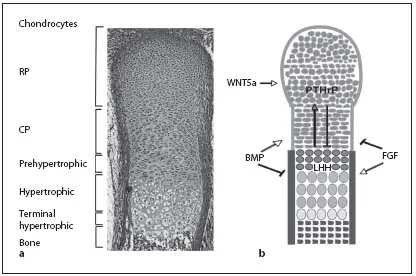
Fig. 2. In the embryonic growth plates, distinct chondrocyte populations can be morphologically distinguished. a Safranin-Weigert staining of a longitudinal section through an E16.5 mouse forelimb showing the characteristic morphology of the cartilage. b Schematic model of a longitudinal section through an embryonic growth plate showing the different chondrocyte subpopulations. Growth factors (BMP, FGF, IHH, PTHrP and WNT5a) can activate (arrows) or inhibit (bars) the formation of distinct chondrocyte populations.
During postnatal development, secondary ossification centers are formed within the zone of the RP chondrocytes (fig. 1d). Between the two ossification centers chondrocytes remain forming the postnatal growth plate. Many studies investigating postnatal growth plate differentiation strongly indicate that the mechanisms controlling the embryonic processes are maintained until adulthood [6].
Longitudinal growth of bones is dependent on the rate of chondrocyte proliferation, the size of the proliferating region and the expansion of the hypertrophic region. During embryonic and postnatal development, the balance between chondrocyte proliferation and differentiation has to be tightly controlled to ensure normal longitudinal bone growth and endochondral bone formation. Both, disturbed chondrocyte proliferation and hypertrophic differentiation, will lead to alterations in bone length and stability.
The sensitivity of the system is reflected by the large number of human chondrodysplasias and the multitude of transgenic mice that show skeletal defects [7]. During the last years, considerable progress has been made in analyzing the signals regulating endochondral ossification. However, the mechanisms by which these signals are translated into gene expression are less well understood. Here, we will summarize some of the relevant signaling pathways and concentrate on recent advances in deciphering the transcriptional network acting downstream of these pathways.
Regulation by Growth Factors
Indian Hedgehog Signaling
One of the key regulators of endochondral bone formation is the secreted growth factor IHH which is expressed in prehypertophic chondrocytes. IHH signals to the proliferating chondrocytes and to the periosteum. In proliferating chondrocytes, IHH signaling directly activates proliferation, especially in CP chondrocytes. In parallel, high IHH signals repress the expansion of the pool of RP chondrocytes, whereas low IHH levels favor its expansion. Furthermore, IHH induces the expression of PTHrP in RP chondrocytes. Activation of the PPR1, expressed in proliferating and prehypertrophic chondrocytes, by PTHrP prevents the onset of hypertrophic differentiation and, thus, the differentiation of IHH-expressing cells (fig. 2b) [8, 9]. By initiating this negative feedback system, IHH regulates the pool of proliferating cells in parallel to the proliferation rate.
Signaling of IHH is mediated by transcription factors of the GLI family, which consist of GLI1, GLI2 and GLI3. GLI1 activates IHH target genes, while GLI2 and GLI3 can function as activators or can be processed into a repressor form [10, 11]. This proteolytic processing is negatively regulated by IHH signaling leading to distinct levels of GLI activator and GLI repressor forms in different chondrocyte subpopulations.
Bone Morphogenetic Protein Signaling
BMPs were identified by their ability to induce ectopic endochondral ossification. Several members of the BMP family, which represents a subgroup of the transforming growth factor family of secreted proteins, and their receptors (BMPRs) are expressed in the cartilage anlagen [12]. BMP signaling is mediated by heteromeric complexes of type I (BM PR1a and BMPR1b) and type II (BMPR2) receptors. Activation of these receptor complexes leads to the phosphorylation of SMAD transcription factors SMAD1, SMAD5 and SMAD8. Phosphorylated SMAD proteins interact with the common mediator SMAD4 to activate transcription of target genes [13]. BMP signaling induces chondrocyte proliferation, whereas loss of both BMPR1a and 1b leads to hypoplastic cartilage anlagen, identifying BMPs as positive regulators of cell cycle progression [4, 14, 15].
Fibroblast Growth Factor Signaling
Like BMPs, FGFs are members of a large family of signaling proteins, which act at multiple steps of the differentiation process. FGF signaling leads to activation of the MAP pathway and phosphorylatio...